Abstract
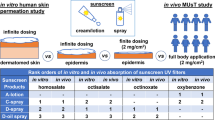
Disodium phenyldibenzimidazole tetrasulfonate (PDT) is a new organic UV filter with hydrophilic properties used in modern sunscreen spray formulations. The aim of this work was to develop and validate an analytical method that can be used to study skin absorption of PDT from sunscreens. Results obtained in vitro for human skin showed a low level of absorption. The proposed in vitro method employs a diffusion cell. Sunscreen lotion was applied onto pretreated human skin, which was then placed in the cell. PDT was collected in a receptor liquid, the surface of which was in contact with the skin. The solutions obtained were diluted appropriately and analyzed by liquid chromatography without any interference. The analytical features of chromatographic determination with fluorimetic detection were suited to this analytical problem, since this method gave a limit of detection of 1 ng ml−1. Phenol red (PR) was used as a marker to check the skin integrity, and a sensitive method based on sequential injection on-line solid-phase extraction coupled with spectrophotometric detection was developed for determining this marker in the receptor liquid in order to screen the cells.




Similar content being viewed by others
References
Benson HA (2000) Am J Clin Dermatol 1:217–224
Benech-Kieffer F, Meuling WJ, Leclerc C, Roza L, Leclaire J, Nohynek G (2003) Skin Pharmacol Appl Skin Physiol 16:343–355
Schauder S, Ippen H (1997) Contact Dermatitis 37:221–232
Berne B, Ros AM (1998) Contact Dermatitis 38:61–64
Schinicit T, Ring J, Abeck D (1998) Dermatology 196:354–357
Darvay A, White IR, Rycroft RJ, Jones AB, Hawk JL, McFadden JP (2001) Br J Dermatol 145:597–601
Okereke CS, Abdel-Rhaman MS, Friedrnan MA (1994) Toxicol Lett 73:113–122
Gustavsson Gonzalez H, Farbrot A, Larko O (2002) Clin Exp Dermatol 27:691–694
Abdel-Nabi IM, Kadry AM, Davis RA, Abel-Rahman MS (1992) J Appl Toxicol 12:255–259
Okereke CS, Kadry AM, Abdel-Rahman MS, Davis RA, Friedman MA (1993) Drug Metab Dispos 21:788–791
Kadry AM, Okereke CS, Abdel-Rahman MS, Friedman MA, Davis RA (1995) J Appl Toxicol 15:97–102
Chatelain E, Gabard B, Surber C (2003) Skin Pharmacol Appl Skin Physiol 16:28–35
Wissing SA, Muller RH (2002) J Control Release 81:225–233
Fernandez C, Nielloud F, Fortune R, Vian L, Marti-Mestres G (2002) J Pharm Biomed Anal 28:57–63
Couteau C, Perez Cudell N, Connan AE, Coiffard LJ (2001) Int J Pharm 222:153–157
Fernandez C, Marti-Mestres G, Ramos J, Maillols H (2000) J Pharm Biomed Anal 24:155–165
Treffel P, Gabard B (1996) Pharm Res 13:770–774
Laugel C, Do Nascimento C, Ferrier D, Marty JP, Baillet A (2001) Appl Spectr 55:1173–1180
Cambon M, Issachar N, Castelli D, Robert C (2001) J Cosmet Sci 52:1–11
Gottbrath S, Gruenefeld J, Mueller-Goymann CC (2003) Trends Clin Exper Dermatol 1:70–77
Sarveiya V, Risk S, Benson HAE (2004)803:225–231
Felix A, Hall BJ, Brodbelt JS (1998) Anal Chem Acta 371:195–203
Vidal MT, Chisvert A, Salvador A (2003) Talanta 59:591–599
Hagedorn-Leweke U, Lippold BC (1995) Pharm Res 12:1354–1360
Marginean-Lazar G, Baillet A, Fructus AE, Arnaud-Battandier J, Ferrier D, Marty JP (1996) Drug Cosmet Ind 158:50–58
Walters KA, Brain KR, Howes D, James VJ, Kraus AL, Teetsel NM, Toulon M, Watkinson AC, Gettings SD (1997) Food Chem Toxicol 35:1219–1225
Pottard G, Laugel C, Baillet A, Schaefer H, Marty JP (1999) Int J Pharm 189:249–260
Jiang R, Roberts MS, Collins DM, Benson HA (1999) Br J Clin Pharmacol 48:635–637
Aghazarian V, Tchiakpe L, Reynier JP, Gayte-Sorbier A (1999) Drug Dev Ind Pharm 25:1277–1282
Fernandez C, Marti-Mestres G, Mestres JP, Maillols H (2000) J Pharm Biomed Anal 22:393–402
Pottard G, LaugelC, Schaefer H, Marty JP (2000) Skin Pharm Appl Skin Physiol 13:336–344
Jiménez MM, Pelletier J, Bobin MF, Martini MC (2004) Int J Pharm 272:45–55
Simeoni S, Scalia S, Benson AE (2004) Int J Pharm 280:163–171
EC (2003) Seventh amendment of Directive 76/768/EEC on Cosmetic Products, Directive 2003/15/EC. European Communities, Brussels
Balaguer A, Chisvert A, Salvador A (2006) J Pharm Biomed Anal 40:922–927
Scott PC, Walker M, Dugard PH (1986) J Soc Cosm Chem 37:35–41
Borrás-Blasco J, Díez-Sales O, López A, Herráez M (2004) Int J Pharm 269:121–129
Diez-Sales O, Garrigues TM, Herraez J.V, Belda R, Martin-Villodre A, Herraez M (2005) J Pharm Sci 94:1039–1047
Salvador A, Chisvert A (2005) Anal Chim Acta 537:15–24
Acknowledgements
The authors would like to express their heartfelt gratitude to the Spanish Analytical Chemistry Society for the prize for the best work in the area of bioanalysis, awarded during the 11th Instrumental Analysis Conference (Barcelona, November 2005). A. Salvador, A. Chisvert and A. Balaguer acknowledge the financial support of the Spanish Ministry of Science and Education for our research project on the development of analytical methods to study the efficacy and safety of sunscreen products (BQU2003-00015). A. Balaguer is also grateful for his grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balaguer, A., Salvador, A., Chisvert, A. et al. A liquid chromatography–fluorimetric method for the in vitro estimation of the skin penetration of disodium phenyldibenzimidazole tetrasulfonate from sunscreen formulations through human skin. Anal Bioanal Chem 385, 1225–1232 (2006). https://doi.org/10.1007/s00216-006-0344-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-006-0344-2