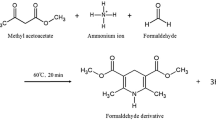
Abstract
A high-performance liquid chromatography (HPLC) method for the determination of acetaldehyde in fuel ethanol was developed. Acetaldehyde was derivatized with 0.900 mL 2,4-dinitrophenylhydrazine (DNPHi) reagent and 50 μL phosphoric acid 1 mol L−1 at a controlled room temperature of 15°C for 20 min. The separation of acetaldehyde-DNPH (ADNPH) was carried out on a Shimadzu Shim-pack C18 column, using methanol/LiCl(aq) 1.0 mM (80/20, v/v) as a mobile phase under isocratic elution and UV–Vis detection at 365 nm. The standard curve of ADNPH was linear in the range 3–300 mg L−1 per injection (20 μL) and the limit of detection (LOD) for acetaldehyde was 2.03 μg L−1, with a correlation coefficient greater than 0.999 and a precision (relative standard deviation, RSD) of 5.6% (n=5). Recovery studies were performed by fortifying fuel samples with acetaldehyde at various concentrations and the results were in the range 98.7–102%, with a coefficient of variation (CV) from 0.2% to 7.2%. Several fuel samples collected from various gas stations were analyzed and the method was successfully applied to the analysis of acetaldehyde in fuel ethanol samples.






Similar content being viewed by others
References
Van den Bergh V, Coeckelberghs H, Vankerckhoven H, Compernolle F, Vinckier C (2004) Anal Bioanal Chem 379:484–494
Cao XO, Zhang ZY, Zhang XR (2004) Sensor Actuat B–Chem 99:30–35
Sugaya N, Sakurai K, Nakagawa T, Onda N, Onodera S, Morita M, Tezuka M (2004) Anal Sci 20:865–870
Schuette F, Park YS, Lee DS (2004) Int J Environ An Ch 84:355–365
Williams PRD (2004) Int Sugar J 106:151
Pereira EA, Rezende MOO, Tavares MFM (2004) J Sep Sci 27:28–32
Ho SSH, Yu JZ (2004) Environ Sci Technol 38:862–870
Liggio J, McLaren R (2003) Int J Environ An Ch 83:819–835
Correa SM, Martins EM, Arbilla G (2003) Atm Environ 37:23–29
Possanzini M, Di Palo V, Cecinato A (2003) Atm Environ 37:1309–1316
Pereira EA, Carrilho E, Tavares MFM (2002) J Chromatogr A 979:409–416
Ho KF, Lee SC, Chiu GMY (2002) Atm Environ 36:57–65
Levart A, Veber M (2001) Chemosphere 44:701–708
Nguyen HTH, Takenaka N, Bandow H, Maeda Y, de Oliva ST, Botelho MMF, Tavares TM (2001) Atm Environ 35:3075–3083
Grosjean E, Green PG, Grosjean D (1999) Anal Chem 71:1851–1861
Lindahl R, Levin JO, Martensson M (1996) Analyst 121:1177–1181
Tsai CF, Shiau HW, Lee SC, Chou SS (2003) J Food Drug Anal 11:46–52
Pereira EA, Cardoso AA, Tavares MFM (2003) Electrophoresis 24:700–706
Sugaya N, Nakagawa T, Sakurai K, Morita M, Onodera S (2001) J Health Sci 47:21–27
Houdier S, Legrand M, Boturyn D, Croze S, Defrancq E, Lhomme J (1999) Anal Chim Acta 382:253–263
Asthana A, Bose D, Kulshrestha S, Pathak SP, Sanghi SK, Kok WT (1998) Chromatographia 48:807–810
de Andrade JB, Reis JN, Rebouças MV, Pinheiro HLC, Andrade MV (1996) Quim Nova 15:144–148
Ortega C, Lopez R, Cacho J, Ferreira V (2001) J Chromatogr A 923:205–214
Kelly J, Chapman S, Brereton P, Bertrand A, Guillou C, Wittkowski R (1999) J AOAC Int 82:1375–1388
Franco DW, Keukeleire DD, Nascimento RF, Marques JC, Neto BSL (1997) J Chromatogr A 782:13–23
Priego-Lopez E, de Castro MDL (2002) J Chromatogr A 976:399–407
Papaefstathiou I, Bilitewski U, de Castro MDL (1997) Fresen J Anal Chem 357:1168–1173
Yasuhara A, Kawada K, Shibamoto T (1998) J Agric Food Chem 46:2664–2670
Miyake T, Shibamoto T (1993) J Agric Food Chem 41:1968–1970
Shiomi K (1991) J High Res Chromatogr 14:136–137
Luong J, Sieben L, Fairhurst M, de Zeeuw J (1996) J High Res Chromatogr 19:591–594
Miyake T, Shibamoto T (1998) J Chromatogr B 719:213–216
Ohata H, Otsuka M, Ohmori S (1997) J Chromatogr B 693:297–305
Ebeler SE, Clifford AJ, Shibamoto T (1997) J Chromatogr B 702:211–215
Stien G, Blanchard F, Rondags E, Marc I (1999) Lait 79:615–624
van Aardt M, Duncan SE, Bourne B, Marcy JE, Long TE, Hackney CR, Heisey C (2001) J Agric Food Chem 49:1377–1381
Dong JZ, Moldovcanu SC (2004) J Chromatogr A1027:25–35
Seila RL, Main HH, Arriaga JL, Martinez G, Ramadan A (2001) Sci Total Environ 276:153–169
Shiraishi T, Soma Y, Ishitani O, Sakamoto K (2001) J Environ Monitor 3:654–660
Uchiyama S, Ando M, Aoyagi S (2003) J Chromatogr A 996:95–102
Smyth MR, Hayes PJ, Mcmurrough I (1987) Analyst 112:1205–1207
Zurek G, Buldt A, Karst U (2000) Fresen J Anal Chem 366:781–791
Komazaki Y, NaritaY, Tanaka S (1998) Analyst 123:2343–2349
Ma WD, Klemm WR (1997) Alcohol 14:469–472
de Andrade JB, Andrade MV, Pinheiro HLC (1998) J Braz Chem Soc 9:219–223
Possanzini M, Di Palo V, Cecinato A (2003) Atm Environ 37:1309–1316
Kiba N, Yagi R, Sun L, Tachibana M, Tani K, Koizumi H, Suzuki T (2000) J Chromatogr A 886:83–87
Komazaki Y, Hiratsuka M, Narita Y, Tanaka S, Fujita T (1999) Fresen J Anal Chem 363:686–695
de Andrade JB, de Andrade MV (1999) Am Lab 31:22
Kozutsumi D, Arita M, Kawashima A, Adachi M, Takami M (2002) J Chromatogr Sci 40:477–482
Peng QT, Hu WX, Hou XJ (2002) Chin Chem Lett 13:1199–1202
Sakuragawa A, Yoneno T, Inowe K, Okutani T (1999) J Chromatogr A 844:403–408
Fung K, Grosjean D (1981) Anal Chem 53:168–171
Coutrim MX, Nakamura LA, Collins CH (1993) Chromatographia 37:185–190
de Andrade JB, Tanner RL (1992) Atm Environ 26:819–825
de Andrade JB, Pinheiro HLC, de Andrade MV (1993) Int J Environ An Ch 52:49–56
Schuette F, Park YS, Lee DS (2004) Int J Environ An Ch 84:355–365
Liggio J, McLaren R (2003) Int J Environ An Ch 83:819–835
Andreini BP, Baroni R, Galimberti E, Sesana G (2000) Microchem J 67:11–19
Lea AGH, Ford GD, Fowler S (2000) Int J Food Sci Tech 35:105–112
Shriner RL, Fugon RC, Curtin DY, Morril TC (1980) The systematic identification of organic compounds. Wiley, New York, p 84
Acknowledgments
We gratefully acknowledge the financial support and fellowships provided by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Agência Nacional do Pétroleo (ANP), and Financiadora de Estudos e Projetos (FINEP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saczk, A.A., Okumura, L.L., Firmino de Oliveira, M. et al. Rapid and sensitive method for the determination of acetaldehyde in fuel ethanol by high-performance liquid chromatography with UV–Vis detection. Anal Bioanal Chem 381, 1619–1624 (2005). https://doi.org/10.1007/s00216-005-3153-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-005-3153-0