Abstract
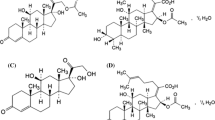
This paper deals with the development of a novel method for simultaneous determination of estradiol, its degradation product estrone, and two preservatives, methylparaben and propylparaben, in the topical preparation Estradiol HBF. After optimization of the analytical conditions the method was validated and applied in studies of the stability of the topical preparation Estrogel HBF. Separation of all these compounds was performed on a Supelco Discovery C18 (250 mm×3.0 mm, 5 μm) analytical column. A mixture of acetonitrile, methanol, and water (23:24:53 v/v) was chosen as mobile phase. UV absorbance at 225 nm was used for detection and quantitation of analytes. The total analysis time was less than 12 min at a flow rate of 0.9 mL min−1. All the compounds were isolated from the topical gel by simple extraction with an acetonitrile solution of hydrocortisone, as internal standard, and using sonication and centrifugation thereafter. The supernatant was injected directly on to the analytical column. The recovery of the procedure was from 96.9 to 100.4%. Validation of method according international guidelines was successfully performed.




Similar content being viewed by others
References
Walters KA, Brain KR, Green DM, James VJ, Watkinson AC, Sands RH (1998) Maturitas 29:189–195
Enriori PJ, Enriori CL (2002) J Steroid Biochem Mol Biol 82:1–6
Andersson TL, Stehle B, Davidsson B, Hőglund P (2000) Maturitas 35:245–252
Jewelewicz R (1997) Fertil Steril 61:1–12
Peltota S, Saarinen-Savolainen P, Kiesvaara J, Suhonen TM, Urtti A (2003) Int J Pharm 254:99–107
Mueck AO, Seeger H, Wallwiener D (2002) Maturitas 43:87–93
Mueck AO, Seeger H, Lippert TH (2002) Maturitas 43:1–10
Hobe G, Shön R, Gornachov N, Katsiya G, Koryakin M, Gesson-Cholat I, Octtel M, Ziemermann H (2002) Steroids 67:883–893
Kimura A, Taguchi M, Arai H, Hiratsuka H, Namba H, Kojima T (2004) Radiat Phys Chem 69:295–301
Lagana A, Fago G, Marino A, Santarelli D (2001) Anal Lett 34:913–926
López de la Alda MJ, Barceló D (2000) J Chromatogr A 892:391–406
Ingrand V, Herry G, Beausse J, de Roubin MR (2003) J Chromatogr A 1020:95-100
Fine DD, Breidenbach P, Price TL, Hutchins SR (2003) J Chromatogr A 1017:167–185
Nakamura S, Sian TH, Daishama S (2001) J Chromatogr A 919:275–282
Xiao XY, McCalley DV, McEvoy J (2001) J Chromatogr A 923:195–204
Cathum S, Sabik H (2001) Chromatographia 53:394–399
Lopez de Alda MJ, Barcelo D (2001) J Chromatogr A 1017:167–185
Penalve A, Polurull E, Borrull R, Marcdé RM (2002) J Chromatogr A 964:153–160
van de Merbel NC, Poelman H (2004) J Pharm Biomed Anal, in press
Yamada H, Yoshizawa K, Hayase T (2002) J Chromatogr B 775:209–213
Nozaki O, Imai YO (1988) Anal Chim Acta 205:255–260
Choi MH, Kim KR, Chung BC (2000) Analyst 125:711–714
Seibert DS, Poole CF (1998) J High Resolut Chromatogr 21:481–490
Salcl B, Biryol I (2002) J Pharm Biomed Anal 28:753–759
Gatti R, Gioia MG, Di Pietra AM, Cavrini V (1998) J Pharm Biomed Anal 18:187–192
Li J, Shah DS (2002) J Chromatogr A 954:159–171
Russel JA, Malcolm RK, Campbell K, Woolfson AD (2000) J Chromatogr B 744:157–163
United States Pharmacopoeial Convention (2002) USP 26, US Pharmacopoeial Convention
Locascio-Brown L, Choquette SJ (1993) Talanta 40:1899–1904
Novakovič J, Pacáková V, Ševčík J, Cserháti T (1996) J Chromatogr B 681:115–123
Medina MB, Schwartz DP (1992) J Chromatogr 581:119–128
Ji AJ, Nunez MF, Machacek D, Ferguson JE, Iossi MF, Kao PC, Landers JP (1995) J Chromatogr B 669:15–26
Su P, Wang YCh, Zhang XX, Sun L, Chang WB (2000) Anal Chim Acta 418:137–143
www.emea.eu.int/htms/human/ich/quality/ichfin.htm
Acknowledgement
The authors gratefully acknowledge the financial support of the Grant Agency of the MSMT of the Czech Republic—FRVS No. 2999/2003—and by the Research project LN00B125 of the Czech Ministry of Education.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nováková, L., Solich, P., Matysová, L. et al. HPLC determination of estradiol, its degradation product, and preservatives in new topical formulation Estrogel HBF. Anal Bioanal Chem 379, 781–787 (2004). https://doi.org/10.1007/s00216-004-2532-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-004-2532-2