Abstract
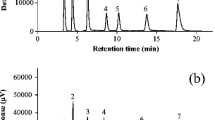
A new and simple method for the determination of fat-soluble vitamins (retinol, α-tocopherol, and β-carotene) in human serum was developed and validated by using liquid chromatography–tandem mass spectrometry with atmospheric pressure chemical ionization (LC-APCI-MS-MS). Different solvent mixtures were tested to obtain deproteinization and extraction of the analytes from the matrix. As a result, a volume of 240 μL of a 1:1 (v/v) ethanol/ethyl acetate mixture added to 60 μL of serum was found to be suitable for both protein precipitation and antioxidants solubilization, giving the best recovery for all three analytes. Deproteinized samples (20 μL) were injected after dilution, without the need for concentration or evaporation to dryness and reconstruction of the sample. Vitamins were separated on a C-8 column using a 95:5 (v/v) methanol/dichloromethane mixture and ionized in the positive-ion mode; detection was performed in the selected-reaction monitoring mode. Linearity of the LC-APCI-MS-MS method was established over 5 orders of magnitude for retinol and α-tocopherol, whereas in the case of β-carotene it was limited to 4 orders. Lower limits of quantitation were 1.7, 2.3, and 4.1 nM for retinol, α-tocopherol, and β-carotene, respectively. Serum concentrations of retinol, α-tocopherol, and α+β-carotene determined in a group of healthy volunteers were 2.48, 38.07, and 0.50 μM, respectively, in samples collected in winter (n=122) and 2.69, 45.88, and 0.90 μM during summer (n=66).



Similar content being viewed by others
References
McCay PB (1985) Annu Rev Nutr 5:323–340
Repine JE, Bast A, Lankhorst I (1997) Am J Resp Crit Care Med 156:341–357
MacNee W, Rahmann I (1999) Am J Respir Crit Care Med 160:S58–S65
Palace VP, Khaper N, Qin Q, Singal PK (1999) Free Rad Biol Med 26:746–761
Mettlin C, Graham S, Swanson M (1979) J Natl Cancer Inst 62:1435–1438
Romieu I, Meneses F, Ramirez M, Ruiz S, Perez Padilla R, Sienra JJ, Gerber M, Grievink L, Dekker R, Walda I, Brunekreef B (1998) Am J Respir Crit Care Med 158:226–232
Schünemann HJ, Grant BJB, Freudenheim JL, Muti P, Browne RW, Drake JA, Klocke RA, Trevisan M (2001) Am J Respir Crit Care Med 163:1246–1255
Vuilleumier J-P, Keller HE, Gysel D, Hunziker F (1983) Internat J Vit Nutr Res 53:265–271
Po ES, Ho JW, Gong BY (1997) J Biochem Biophys Methods 34:99–106
Wang LH, Wang JF (2001) J Pharm Biomed Anal 25:785–793
Gueguen S, Herbeth B, Siest G, Leroy P (2002) J Chromatogr Sci 40:69–76
Khachik F, Spangler CJ, Smith JC Jr, Canfield LM, Steck A, Pfander H (1997) Anal Chem 69:1873–1881
Abahusain MA, Wright J, Dickerson JWT, El-Hazmi MA, Aboul Enein HY (1998) Biomed Chromatogr 12:89–93
van Breemen RB, Nikolic D, Xu X, Xiong Y, van Lieshout M, West CE, Schilling AB (1998) J Chromatogr A 794:245–251
Lauridsen C, Leonard SW, Griffin DA, Liebler DC, McClure TD, Traber MG (2001) Anal Biochem 289:89–95
Hagiwara T, Yasuno T, Funayama K, Suzuki S (1998) J Chromatogr B 708:67–73
Wang Y, Xu X, van Lieshout M, West CE, Lugtenburg J, Verhoeven MA, Creemers AFL, Muhilal, van Breemen RB (2000) Anal Chem 72:4999–5003
van Breemen RB, Huang C-R, Tan Y, Sander LC, Schilling AB (1996) J Mass Spectrom 31:975–981
van Breemen R (1995) Anal Chem 67:2004–2009
Careri M, Lugari MT, Mangia A, Manini P, Spagnoli S (1995) Fres J Anal Chem 351:768–776
Fang L, Pajkovic N, Wang Y, Gu C, van Breemen RB (2003) Anal Chem 75:812–817
Acknowledgements
This work was financially supported by the European Community (Contract No. QLK4-CT-1999–01308) and by the Azienda Ospedaliera di Parma (Italy). The collaboration of Drs Licia Rossi and Chiara Tanzi is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Andreoli, R., Manini, P., Poli, D. et al. Development of a simplified method for the simultaneous determination of retinol, α-tocopherol, and β-carotene in serum by liquid chromatography–tandem mass spectrometry with atmospheric pressure chemical ionization. Anal Bioanal Chem 378, 987–994 (2004). https://doi.org/10.1007/s00216-003-2288-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-003-2288-0