Abstract
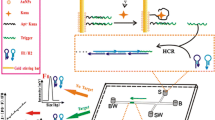
The development of a generic semi-disposable microfluidic biosensor for the highly sensitive detection of pathogens via their nucleic acid sequences is presented in this paper. Disposable microchannels with defined areas for capture and detection of target pathogen RNA sequence were created in polydimethylsiloxane (PDMS) and mounted onto a reusable polymethylmethacrylate (PMMA) stand. Two different DNA probes complementary to unique sequences on the target pathogen RNA serve as the biorecognition elements. For signal generation and amplification, one probe is coupled to dye encapsulated liposomes while the second probe is coupled to superparamagnetic beads for target immobilization. The probes hybridize to target RNA and the liposome–target-bead complex is subsequently captured on a magnet. The amount of liposomes captured correlates directly to the concentration of target sequence and is quantified using a fluorescence microscope. Dengue fever virus serotype 3 sequences and probes were used as a model analyte system to test the sensor. Probe binding and target capture conditions were optimized for sensitivity resulting in a detection limit of as little as 10 amol μL−1 (10 pmol L−1) . Future biosensors will be designed to incorporate a mixer and substitute the fluorescence detection with an electrochemical detection technique to provide a truly portable microbiosensor system.






Similar content being viewed by others
References
Kow C, Koon L, Yin P (2001) J Med Entomol 378:475–479
Laue T, Emmerich P, Schmitz H (1999) J Clin Microbiol 37:2543–2547
Killen H, O'Sullivan M (1993) J Virol Methods 41:135–146
Baeumner A, Schlesinger N, Slutzki N, Romano J, Lee E, Montagna R (2002) Anal Chem 74:1442–1448
Mondesire R, Kozwich D, Johansen K, Gerdes J, Beard S (2000) IVD Magazine, May, 9–14
Yu H, Sethu P, Chan T, Kroutchinina N, Blackwell J, Mastrangelo C, Grodzinski P (2000) Micro Total Analysis Systems Conference, Enschede, Netherlands, May 2000, pp 545–548
Kopp M, de Mello A, Manz A (1998) Science 280:1046–1048
Manz A, Harrison D, Verpoorte E, Fettinger J, Paulus A, Lüdi H, Widmer H (1992) J Chromatogr 593:253–258
Duffy D, McDonald J, Schueller O, Whitesides G (1998) Anal Chem 70:4974–4984
Jingdong X, Locascio L, Gaitan M, Lee C (2000) Anal Chem 72:1930–1933
Martynova L, Locascio L, Gaitan M, Kramer G, Christensen R, MacCrehan W (1997) Anal Chem 69:4783–4789
Ramsay G (1998) Nat Biotech 16:40–44
Edelstein R, Tamanaha C, Sheehan P, Miller M, Baselt D, Whitman L, Colton R (2000) Biosens Bioelectron 14:805
Esch M, Locascio L, Tarley M, Durst R (2001) Anal Chem 73:2952–2958
Taton T, Mirkin C, Letsinger R (2002) Science 289:1756–1760
Cao Y, Jin R, Mirkin C (2002) Science 297:1536–1540
Lee M, Durst R, Wong R (1997) Anal Chim Acta 354:23–28
Esch M, Baeumner A, Durst R (2001) Anal Chem 73:3162–3167
Rule G, Montagna R, Durst R (1996) Clin Chem 42:206–1209
Baeumner A, Cohen R, Miksic V, Min J (2003) Biosens Bioelectron, 8:405–419
Dynal Inc (1998) Biomagnetic techniques in molecular biology, 3 edn, 5 Delaware Drive, Lake Success, NY 11042
Dhawan M, Wise F, Baeumner A (2002) Anal Bioanal Chem 374:421–426
Hartley H, Baeumner A (2003) Anal Bioanal Chem, 376:319–327
Acknowledgements
The authors thank former laboratory colleagues Sui Ping Lee and Nicole Schlesinger for their help with the preparation of liposomes. We thank Dr Jun Min, Cornell University for helpful late-night discussions concerning microchannel design. We also thank Dr Laurie Locascio and Dr Susan Barker of the MicroAnalytical Laboratory, NIST, Gaithersburg, Maryland, USA, for microfabrication training. The authors acknowledge financial support for this project from the department of Biological and Environmental Engineering, Cornell University and the Cooperative State Research, Education and Extension Services (NYC-123404). This work was performed in part at the Cornell Nanfabrication Facility (a member of the National Nanofabrication Users Network) which is supported by the National Science Foundation under Grant ECS-9731293, its users, Cornell University and Industrial Affiliates.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kwakye, S., Baeumner, A. A microfluidic biosensor based on nucleic acid sequence recognition. Anal Bioanal Chem 376, 1062–1068 (2003). https://doi.org/10.1007/s00216-003-2063-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-003-2063-2