Abstract
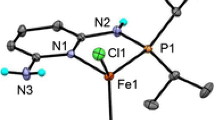
A previous report introduced a new series of cationic nickel(II) complexes ligated by PCP-type pincer ligands featuring a charge-bearing imidazoliophosphine binding moiety and described their catalytic reactivities in hydroamination of nitriles into amidines. Solid-state characterization of the cationic acetonitrile adducts [(R-PIMIOCOP+)Ni(NCMe)(triflate)]+ (R-PIMIOCOP+ = κP,κC,κP-{2-(R2PO),6-(R2PC4H5N2)C6H3}; R = i-Pr, [1]+; Ph, [2]+) carried out in this follow-up study showed a distorted square pyramidal geometry and a Ni–triflate distance that was shorter than the sum of the Ni and O van der Waals radii, features suggestive of an unusual pentacoordination at the Ni(II) center. In contrast, the related aquo adduct [(i-Pr-PIMIOCOP+)Ni(OH2)(triflate)]+, [3]+, displayed a more conventional square planar geometry. Detailed structural comparisons and theoretical analyses conducted on these and related compounds have allowed a thorough examination of the Ni–triflate interactions in this family of complexes. Thus, topological analysis of the electron localization function (ELF) and quantum theory of atoms in molecules (QTAIM) showed that the Ni–triflate interaction is mostly ionic in nature, but has a weak covalence degree. The monosynaptic V(Ni) subvalence basin of nickel is indeed the ELF signature of the covalence degree of the ionic Ni–O bond, which can be quantified by the negative QTAIM energy density at the Ni–O bond critical point and by the absolute value of the ELF covariance 〈σ2(V(O), C(Ni))〉. The ionic character of the Ni–O bond is also reflected in an energy decomposition analysis, showing that this interaction is mostly electrostatic in nature. The computational analyses carried out on this family of complexes provide valuable insight into the character and relative strengths of various Ni–ligand interactions, and allow a number of useful conclusions, including the following: (1) significant Ni–anion interactions at the apical site are observed only with pincer-type ligands featuring at least one cationic imidazoliophosphine binding moiety; (2) these primarily electrostatic Ni–O interactions gain increasing covalence degree when different pincer backbone, co-ligand L, or counter-anions are introduced to enhance the electron deficiency of the Ni(II) center.













Similar content being viewed by others
Notes
The coordination plane in question is the least-squares plane defined by P1, C5, P2, and N3 in the acetonitrile adducts and by P1, C5, P2, O8 in the aquo adduct.
The τ index is commonly used to categorize the geometry of a 5-coordinated species. Defined by the equation (β − α)/60 where β and α (in degrees) are the two largest valence angles of the coordination center (β > α), τ takes numerical values ranging from 0 (for an ideal square pyramidal geometry) to 1 (for an ideal trigonal bipyramidal geometry). The extent of distortion from the two ideal geometries can thus be estimated from the τ value calculated for a given 5-coordinated complex. For a discussion of τ index, see [15].
The complete set of ELF data for complex [1]++ is presented in the Electronic Supporting Information of reference [9].
This approach for estimating the formal oxidation number using ELF analysis was successfully applied to ambiguous cases, and the results were nicely correlated with the X-ray photoelectron spectroscopy results. See [19].
The population of V(Ni) is insensitive to the accuracy level of the ELF analysis (grid size, approximation in the gradient field analysis), the location of the V(Ni) attractor, the volume, and the V(Ni) population depend on the type of double-zeta basis set (6-31G**, 6-31++G**, DGDZVP, …). See Table S1 in SI.
For a classification of various ligands, see [27].
According to Refs. [20, 21], the electrostatic interaction between the metallic cation and the ligand is more polarized or covalent, when the number of monosynaptic basins describing the lone pair of the ligand increases. As a result, because of the differential polarizing field surrounding it, the cation splits its outer-shell density toward the ligands into localization domains called subvalence basins whose volume and population increase as the nature of the interaction becomes more covalent/polarized.
Molekel 4.3 from CSCS: http://www.cscs.ch/molekel/.
Optionally, you may add the following list of authors and contributors:
E.J. Baerends, T. Ziegler, J. Autschbach, D. Bashford, A. Bérces, F.M. Bickelhaupt, C. Bo, P.M. Boerrigter, L. Cavallo, D.P. Chong, L. Deng, R.M. Dickson, D.E. Ellis, M. van Faassen, L. Fan, T.H. Fischer, C. Fonseca Guerra, M. Franchini, A. Ghysels, A. Giammona, S.J.A. van Gisbergen, A.W. Götz, J.A. Groeneveld, O.V. Gritsenko, M. Grüning, S. Gusarov, F.E. Harris, P. van den Hoek, C.R. Jacob, H. Jacobsen, L. Jensen, J.W. Kaminski, G. van Kessel, F. Kootstra, A. Kovalenko, M.V. Krykunov, E. van Lenthe, D.A. McCormack, A. Michalak, M. Mitoraj, S.M. Morton, J. Neugebauer, V.P. Nicu, L. Noodleman, V.P. Osinga, S. Patchkovskii, M. Pavanello, P.H.T. Philipsen, D. Post, C.C. Pye, W. Ravenek, J.I. Rodríguez, P. Ros, P.R.T. Schipper, G. Schreckenbach, J.S. Seldenthuis, M. Seth, J.G. Snijders, M. Solà, M. Swart, D. Swerhone, G. te Velde, P. Vernooijs, L. Versluis, L. Visscher, O. Visser, F. Wang, T.A. Wesolowski, E.M. van Wezenbeek, G. Wiesenekker, S.K. Wolff, T.K. Woo, A.L. Yakovlev.
References
Crabtree RH (2005) The organometallic chemistry of the transition metals, 4th edn. Wiley, Hoboken, p 35
Huheey JE, Keiter EA, Keiter LR (1993) Inorganic chemistry: principles of structure and reactivity, 4th edn. Harper Collins College, New York, p 936
Roddick DM, Zargarian D (2014) Inorg Chim Acta 422:251–264
Hope H, Olmstead MM, Power PP, Viggiano M (1984) Inorg Chem 23:326–330
Stalick JK, Ibers JA (1969) Inorg Chem 8:1084–1090
Klein HF, Dal A, Jung T, Flörke U, Haupt HJ (1998) Eur J Inorg Chem 12:2027–2032
Klein HF, Zwiener M, Petermann A, Jung T, Cordier G, Hammerschmitt B, Flörke U, Haupt HJ, Dartiguenave Y (1994) Chem Ber 127:1569–1578
Vabre B, Canac Y, Duhayon C, Chauvin R, Zargarian D (2012) Chem Commun 48:10446–10448
Vabre B, Canac Y, Lepetit C, Duhayon C, Chauvin R, Zargarian D (2015) Chem Eur J 21:17403–17414
Wu S, Li X, Xiong Z, Xu W, Lu Y, Sun H (2013) Organometallics 32:3227–3237
Kozhanov KA, Bubnov MP, Vavilina NN, Efremova LY, Fukin GK, Cherkasov VK, Abakumov GA (2009) Polyhedron 28:2555–2558
Kozhanov KA, Bubnov MP, Cherkasov VK, Vavilina NV, Efremova LY, Artyushin OI, Odinets IL, Abakumov GA (2008) Dalton Trans 21:2849–2853
Kozhanov KA, Bubnov MP, Cherkasov VK, Fukin GK, Abakumov GA (2003) Chem Commun 20:2610
Kozhanov KA, Bubnov MP, Cherkasov VK, Fukin GK, Abakumov GA (2004) Dalton Trans 18:2957–2962
Addison AW, Rao NT, Reedijk J, van Rijn J, Verschoor GC (1984) J Chem Soc Dalton Trans (7):1349–1355
Zargarian D, Castonguay A, Spasyuk DM (2013) In: van Koten G, Milstein D (eds) Topics in organometallic chemistry, vol 40. Springer, Berlin, pp 131–174
Salah A, Offenstein C, Zargarian D (2011) Organometallics 30:5352–5364
Noury S, Krokidis X, Fuster F, Silvi B (1999) Comput Chem 23:597–604
Andres J, Feliz M, Fraxedas J, Hernandez V, Lopez-Navarrete JT, Llusar R, Sauthier G, Sensato FR, Silvi B, Bo C, Campanera JM (2007) Inorg Chem 46:2159–2166
De Courcy B, Dognon J-P, Clavaguéra C, Gresh N, Piquemal J-P (2011) Int J Quant Chem 111:1213–1221
de Courcy B, Pedersen LG, Parisel O, Gresh N, Silvi B, Pilmé J, Piquemal J-P (2010) J Chem Theor Comput 6:1048–1063
Bader RFW (1990) Atoms in molecules. Clarendon Press, Oxford
Bader RFW, Essen H (1984) J Chem Phys 80:1943–1960
Bianchi R, Gervasio G, Marabello D (2000) Inorg Chem 39:2360–2366
Lepetit C, Fau P, Fajerwerg K, Kahn ML, Silvi B (2017) Coord Chem Rev 345:150–181
Macchi P, Proserpio DM, Sironi A (1998) J Am Chem Soc 120:13429–13435
Green MLH (1995) A new approach to the formal classification of the covalent compounds of the elements. J Organomet Chem 500:127–148
Espinosa E, Alkorta I, Elguero J, Molins E (2002) J Chem Phys 117:5529–5542
Espinosa E, Molins E, Lecomte C (1998) Chem Phys Lett 285:170–173
Espinosa E, Alkorta I, Rozas I, Elguero J, Molins E (2001) Chem Phys Lett 336:457–461
Ziegler T, Rauk A (1979) Inorg Chem 18:1558–1565
Ziegler T, Rauk A (1979) Inorg Chem 18:1755–1759
Bickelhaupt FM, Baerends EJ (2000) In: Lipkowitz KB, Boyd DB (eds) Reviews in computational chemistry, vol 15. Wiley, New York, pp 1–86
Jacobsen H, Correa A, Poater A, Costabile C, Cavallo L (2009) Coord Chem Rev 253:687–703
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, revision D.01. Gaussian Inc., Wallingford
Ehlers AW, Böhme M, Dapprich S, Gobbi A, Höllwarth A, Jonas V, Köhler KF, Stegmann R, Veldkamp A, Frenking G (1993) Chem Phys Lett 208:111–114
Becke AD, Edgecombe KE (1990) J Chem Phys 92:5397–5403
Silvi B, Savin A (1994) Nature 371:683–686
Keith TA (2016) AIMAll (version 16.01.09). TK Gristmill Software, Overland Park
Spackman MA (2015) Cryst Growth Des 15:5624–5628
Nelyubina YV, Antipin MY, Lyssenko KA (2010) Russ Chem Rev 79:167–187
Gatti C (2005) Z Kristallogr Cryst Mater 220:399–457
Valyaev DA, Brousses R, Lugan N, Fernàndez I, Sierra MA (2011) Chem Eur J 17:6602–6605
Borissova AO, Korlyukov AA, Antipin MY, Lyssenko KA (2008) J Phys Chem A 112:11519–11522
Puntus LN, Lyssenko KA, Antipin MY, Bünzli JCG (2008) Inorg Chem 47:1105–11107
Poater J, Duran M, Sola M, Silvi B (2005) Chem Rev 105:3911–3947
Silvi B, Gillespie RJ, Gatti C (2013) Compr Inorg Chem II 9:187–226
Lepetit C, Silvi B, Chauvin R (2003) J Phys Chem A 107:464–473
Silvi B (2004) Phys Chem Chem Phys 6:256–260
te Velde G, Bickelhaupt FM, van Gisbergen SJA, Fonseca Guerra C, Baerends EJ, Snijders JG, Ziegler T (2001) J Comput Chem 22:931–967
Fonseca Guerra C, Snijders JG, te Velde G, Baerends EJ (1998) Theor Chem Acc 99:391–403
ADF2013, SCM, Theoretical chemistry, Vrije Universiteit, Amsterdam, The Netherlands. http://www.scm.com
Van Lenthe E, Baerends EJ (2003) J Comput Chem 24:1142–1156
Pye CC, Ziegler T (1999) Theor Chem Acc 101:396–408
Acknowledgements
The theoretical studies were performed using HPC resources from CALMIP (Grant 2013-2018 [0851]]) and from GENCI-[CINES/IDRIS] (Grant 2013-2018 [085008]). The authors gratefully acknowledge the financial support provided by NSERC (Discovery grant to DZ) and FRQNT (Ph.D. fellowship to BV). The Direction des Relations Internationales of Université de Montréal and Université Toulouse 3-Paul Sabatier are gratefully acknowledged for the travel grants that made this collaborative project possible. The authors would like to thank Professor Bernard Silvi for fruitful discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published as part of the special collection of articles “CHITEL 2017-Paris-France.”
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lepetit, C., Vabre, B., Canac, Y. et al. Pentacoordinated, square pyramidal cationic PCP Ni(II) pincer complexes: ELF and QTAIM topological analyses of nickel–triflate interactions. Theor Chem Acc 137, 141 (2018). https://doi.org/10.1007/s00214-018-2332-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-018-2332-y