Abstract
The aim of this work was the calibration and validation of mathematical models based on a quantitative structure–activity relationship approach to discriminate sweet, tasteless and bitter molecules. The sweet-tasteless and the sweet-bitter datasets included 566 and 508 compounds, respectively. A total of 3763 conformation-independent Dragon molecular descriptors were calculated and subsequently reduced through both unsupervised reduction and supervised selection coupled with the k-nearest neighbors classification technique. A model based on nine descriptors was retained as the optimal one for sweet and tasteless molecules, while a model based on four descriptors was calibrated for the sweetness-bitterness dataset. Models were properly validated through cross-validation and external test sets. The applicability domain of models was investigated, and the interpretation of the role of the molecular descriptors in classifying sweet and non-sweet tastes was evaluated. The classification and the performance of the models presented in this paper are simple but accurate. They are based on a relatively small number of descriptors and a straightforward classification approach. The results presented here indicate that the proposed models can be used to accurately select new compounds as potential sweetener candidates.




Similar content being viewed by others
References
Shallenberger RS (1993) Taste chemistry. Springer Science & Business Media, Berlin
Hugot E, Jenkins GH (1972) Handbook of cane sugar engineering, vol 114. Elsevier, Philadelphia
Asadi M (2006) Beet-sugar handbook. Wiley, NewYork
Birch GG (1999) Modulation of sweet taste. BioFactors 9(1):73–80
deMan JM (1999) Principles of food chemistry, 3rd edn. Berlin, Springer
Oertly E, Myers RG (1919) A new theory relating constitution to taste. Simple relations between the constitution of aliphatic compounds and their sweet taste. J Am Chem Soc 41(6):855–867
Shallenberger RS, Acree TE (1967) Molecular theory of sweet taste. Nature 216:480–482
Kier LB (1972) A molecular theory of sweet taste. J Pharm Sci 61(9):1394–1397
Nofre C, Tinti J-M (1996) Sweetness reception in man: the multipoint attachment theory. Food Chem 56(3):263–274
Ellis JW (1995) Overview of sweeteners. J Chem Educ 72(8):671
Katritzky AR, Lobanov VS, Karelson M (1995) QSPR: the correlation and quantitative prediction of chemical and physical properties from structure. Chem Soc Rev 24:279–287
Trinajstic N (1992) Chemical graph theory. CRC Press, Boca Raton
Diudea MV (2001) QSPR/QSAR studies by molecular descriptors. Nova Science Publishers, New York
Todeschini R, Consonni V (2009) Molecular descriptors for chemoinformatics, vol 2. Wiley-VCH, Weinheim
Iwamura H (1980) Structure-taste relationship of perillartine and nitro-and cyanoaniline derivatives. J Med Chem 23(3):308–312
van der Wel H, van der Heijden A, Peer H (1987) Sweeteners. Food Rev Int 3(3):193–268
Kier LB (1980) Molecular structure influencing either a sweet or bitter taste among aldoximes. J Pharm Sci 69(4):416–419
Takahashi Y, Miyashita Y, Tanaka Y, Abe H, Sasaki S (1982) A consideration for structure-taste correlations of perillartines using pattern-recognition techniques. J Med Chem 25(10):1245–1248
Takahashi Y, Abe H, Miyashita Y, Tanaka Y, Hayasaka H, Sasaki SI (1984) Discriminative structural analysis using pattern recognition techniques in the structure-taste problem of perillartines. J Pharm Sci 73(6):737–741
Miyashita Y, Takahashi Y, Takayama C, Ohkubo T, Funatsu K, Sasaki S-I (1986) Computer-assisted structure/taste studies on sulfamates by pattern recognition methods. Anal Chim Acta 184:143–149
Miyashita Y, Takahashi Y, Takayama C, Sumi K, Nakatsuka K, Ohkubo T, Abe H, Sasaki S (1986) Structure-taste correlation of L-aspartyl dipeptides using the SIMCA method. J Med Chem 29(6):906–912
Okuyama T, Miyashita Y, Kanaya S, Katsumi H, S-i Sasaki, Randić M (1988) Computer assisted structure-taste studies on sulfamates by pattern recognition method using graph theoretical invariants. J Comput Chem 9(6):636–646
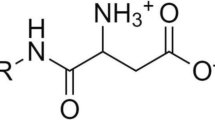
Spillane WJ, McGlinchey G (1981) Structure-activity studies on sulfamate sweeteners II: semiquantitative structure-taste relationship for sulfamate (RNHSO3 −) sweeteners-the role of R. J Pharm Sci 70(8):933–935
Spillane WJ, McGlinchey G, Muircheartaigh IÓ, Benson GA (1983) Structure-activity studies on sulfamate sweetners III: structure-taste relationships for heterosulfamates. J Pharm Sci 72(8):852–856
Spillane WJ, Sheahan MB (1989) Semi-quantitative and quantitative structure-taste relationships for carboand hetero-sulphamate (RNHSO3 −) sweeteners. J Chem Soc, Perkin Trans 2(7):741–746
Spillane WJ, Sheahan M (1991) Structure-taste relationships for sulfamate sweeteners (RNHSO3 −). Phosphorus Sulfur Silicon Relat Elem 59(1–4):255–258
Spillane WJ, Sheahan MB, Ryder CA (1993) Synthesis and taste properties of sodium disubstituted phenylsulfamates. Structure-taste relationships for sweet and bitter/sweet sulfamates. Food Chem 47(4):363–369
Drew MGB, Wilden GRH, Spillane WJ, Walsh RM, Ryder CA, Simmie JM (1998) Quantitative structure-activity relationship studies of sulfamates RNHSO3Na: distinction between sweet, sweet-bitter, and bitter molecules. J Agric Food Chem 46(8):3016–3026
Spillane WJ, Ryder CA, Curran PJ, Wall SN, Kelly LM, Feeney BG, Newell J (2000) Development of structure-taste relationships for sweet and non-sweet heterosulfamates. J Chem Soc Perkin Trans 2(7):1369–1374
Spillane WJ, Feeney BG, Coyle CM (2002) Further studies on the synthesis and tastes of monosubstituted benzenesulfamates. A semi-quantitative structure-taste relationship for the meta-compounds. Food Chem 79(1):15–22
Spillane WJ, Kelly LM, Feeney BG, Drew MG, Hattotuwagama CK (2003) Synthesis of heterosulfamates. Search for structure-taste relationships. Arkivoc 7:297–309
Kelly DP, Spillane WJ, Newell J (2005) Development of structure-taste relationships for monosubstituted phenylsulfamate sweeteners using classification and regression tree (CART) analysis. J Agric Food Chem 53(17):6750–6758
Spillane WJ, Kelly DP, Curran PJ, Feeney BG (2006) Structure-taste relationships for disubstituted phenylsulfamate tastants using classification and regression tree (CART) Analysis. J Agric Food Chem 54(16):5996–6004
Spillane WJ, Coyle CM, Feeney BG, Thompson EF (2009) Development of structure-taste relationships for thiazolyl-, benzothiazolyl-, and thiadiazolylsulfamates. J Agric Food Chem 57(12):5486–5493
Spillane WJ (1993) Structure taste studies of sulphamates. In: Mathlouthi M, Kanters JA, Birch GG (eds) Sweet-taste chemoreception. Elsevier Science Publishers, Philadelphia, p 283
Spillane WJ, Ryder CA, Walsh MR, Curran PJ, Concagh DG, Wall SN (1996) Sulfamate sweeteners. Food Chem 56(3):255–261
Walters DE (2006) Analysing and predicting properties of sweet-tasting compounds. In: Spillane WJ (ed) Optimising sweet taste in foods. pp 283–291
Rojas C, Duchowicz PR, Pis Diez R, Tripaldi P (2016) Applications of quantitative structure-relative sweetness relationships in food chemistry. In: Mercader AG, Duchowicz PR, Sivakumar PM (eds) Chemometrics applications and research: QSAR in medicinal chemistry. CRC Press, Taylor & Francis Group, pp 317–339
van der Heijden A (1997) Historical overview on structure-activity relationships among sweeteners. Pure Appl Chem 69(4):667–674
Spillane W, Malaubier J-B (2014) Sulfamic acid and its N-and O-substituted derivatives. Chem Rev 114(4):2507–2586
Organisation for Economic Co-operation and Development (2007) Guidance document on the validation of (quantitative)structure-activity relationships [(Q)SAR] models. OECD Publishing, Paris
Arnoldi A, Bassoli A, Borgonovo G, Drew MG, Merlini L, Morini G (1998) Sweet isovanillyl derivatives: synthesis and structure-taste relationships of conformationally restricted analogues. J Agric Food Chem 46(10):4002–4010
Arnoldi A, Bassoli A, Borgonovo G, Merlini L (1995) Synthesis and sweet taste of optically active (−)-haematoxylin and of some (±)-haematoxylin derivatives. J Chem Soc Perkin Trans 1(19):2447–2453
Arnoldi A, Bassoli A, Borgonovo G, Merlini L, Morini G (1997) Synthesis and structure-activity relationships of sweet 2-benzoylbenzoic acid derivatives. J Agric Food Chem 45(6):2047–2054
Arnoldi A, Bassoli A, Merlini L (1996) Progress in isovanillyl sweet compounds. Food Chem 56(3):247–253
Arnoldi A, Bassoli A, Merlini L, Ragg E (1991) Isovanillyl sweeteners. Synthesis, conformational analysis, and structure-activity relationship of some sweet oxygen heterocycles. J Chem Soc Perkin Trans 2(9):1399–1406
Arnoldi A, Bassoli A, Merlini L, Ragg E (1993) Isovanillyl sweeteners. Synthesis and sweet taste of sulfur heterocycles. J Chem Soc Perkin Trans 1(12):1359–1366
Bassoli A, Borgonovo G, Drew MG, Merlini L (2000) Enantiodifferentiation in taste perception of isovanillic derivatives. Tetrahedron Asymmetry 11(15):3177–3186
Bassoli A, Drew MGB, Hattotuwagama CK, Merlini L, Morini G, Wilden GRH (2001) Quantitative structure-activity relationships of sweet isovanillyl derivatives. Quant Struct-Act Relat 20(1):3–16
Belitz H-D, Grosch W, Schieberle P (2009) Food chemistry, 4th edn. Springer-Verlag, Heidelberg
Nanayakkara NPD, Hussain RA, Pezzuto JM, Soejarto DD, Kinghorn AD (1988) An intensely sweet dihydroflavonol derivative based on a natural product lead compound. J Med Chem 31(6):1250–1253
O’Brien-Nabors L (2001) Alternative sweeteners, 3rd edn. New York, Marcel Dekker Inc
Yamato M, Hashigaki K (1979) Chemical structure and sweet taste of isocoumarins and related compounds. Chem Senses 4(1):35–47
Yang X, Chong Y, Yan A, Chen J (2011) In-silico prediction of sweetness of sugars and sweeteners. Food Chem 128(3):653–658
Zhong M, Chong Y, Nie X, Yan A, Yuan Q (2013) Prediction of sweetness by multilinear regression analysis and support vector machine. J Food Sci 78(9):S1445–S1450
Paulus K, Reisch AM (1980) The influence of temperature on the threshold values of primary tastes. Chem Senses 5(1):11–21
Open Babel, Open Babel: The Open Source Chemistry Toolbox. http://openbabel.org/
Berthold M, Cebron N, Dill F, Gabriel T, Kötter T, Meinl T, Ohl P, Sieb C, Thiel K, Wiswedel B (2008) KNIME: the konstanz information miner. In: Preisach C, Burkhardt H, Schmidt-Thieme L, Decker R (eds) Data analysis, machine learning and applications. Studies in classification, data analysis, and knowledge organization. Springer, Berlin Heidelberg, pp 319–326
Hypercube, Inc., HyperChem. http://www.hyper.com
TALETE, srl., Dragon (version 6) (2015). Software for Molecular Descriptor Calculation, http://www.talete.mi.it/
Pearlman RS (1993) 3D molecular structures: generation and use in 3D searching. In: Kubinyi H (ed) 3D QSAR in drug design. Theory and applications, Springer Science & Business Media, pp 41–79
Doweyko AM (2004) 3D-QSAR illusions. J Comput Aided Mol Des 18(7–9):587–596
Hechinger M, Leonhard K, Marquardt W (2012) What is wrong with quantitative structure-property relations models based on three-dimensional descriptors? J Chem Inf Model 52(8):1984–1993
Kowalski B, Bender C (1972) k-Nearest neighbor classification rule (pattern recognition) applied to nuclear magnetic resonance spectral interpretation. Anal Chem 44(8):1405–1411
Ballabio D, Consonni V, Mauri A, Claeys-Bruno M, Sergent M, Todeschini R (2014) A novel variable reduction method adapted from space-filling designs. Chemometr Intell Lab Syst 136:147–154
Leardi R, Gonzalez AL (1998) Genetic algorithms applied to feature selection in PLS regression: how and when to use them. Chemometr Intell Lab Syst 41(2):195–207
Ballabio D, Consonni V (2013) Classification tools in chemistry. Part 1: linear models. PLS-DA. Anal Methods 5(16):3790–3798
Wold S, Esbensen K, Geladi P (1987) Principal component analysis. Chemometr Intell Lab Syst 2(1):37–52
Jolliffe IT (1986) Principal component analysis. Springer Science + Business Media, Berlin
Krzanowski W (1988) Principles of multivariate analysis: a user’s perspective. Oxford University Press, Oxford
Bro R, Smilde AK (2014) Principal component analysis. Anal Methods 6(9):2812–2831
Sahigara F, Mansouri K, Ballabio D, Mauri A, Consonni V, Todeschini R (2012) Comparison of different approaches to define the applicability domain of QSAR models. Molecules 17(5):4791–4810
Sahigara F, Ballabio D, Todeschini R, Consonni V (2013) Defining a novel k-nearest neighbours approach to assess the applicability domain of a QSAR model for reliable predictions. J Cheminfor 5:27
Cassotti M, Consonni V, Mauri A, Ballabio D (2014) Validation and extension of a similarity-based approach for prediction of acute aquatic toxicity towards Daphnia magna. SAR QSAR Environ Res 25(12):1013–1036
Cassotti M, Ballabio D, Consonni V, Mauri A, Tetko IV, Todeschini R (2014) Prediction of acute aquatic toxicity toward Daphnia magna by using the GA-kNN method. Altern Lab Anim 42:31–41
Cassotti M, Ballabio D, Todeschini R, Consonni V (2015) A similarity-based QSAR model for predicting acute toxicity towards the fathead minnow (Pimephales promelas). SAR QSAR Environ Res 26(3):217–243
Ballabio D (2015) A MATLAB toolbox for principal component analysis and unsupervised exploration of data structure. Chemometr Intell Lab Syst 149:1–9
MathWorks: Natick, MatLab (version 7.13.0.564) (2011). http://www.mathworks.com
Carhart RE, Smith DH, Venkataraghavan R (1985) Atom pairs as molecular features in structure-activity studies: definition and applications. J Chem Inf Comput Sci 25(2):64–73
Birch GG, Karim R, Lopez A (1994) Novel aspects of structure-activity relationships in sweet taste chemoreception. Food Qual Prefer 5(1):87–93
Rojas C, Tripaldi P, Duchowicz PR (2016) A new QSPR study on relative sweetness. Int J Quant Struct Prop Relatsh 1(1):76–90
Ghose AK, Viswanadhan VN, Wendoloski JJ (1998) Prediction of hydrophobic (lipophilic) properties of small organic molecules using fragmental methods: an analysis of ALOGP and CLOGP methods. J Phys Chem A 102(21):3762–3772
Baek N-I, Chung M-S, Shamon L, Kardono LBS, Tsauri S, Padmawinata K, Pezzuto JM, Soejarto DD, Kinghorn AD (1993) Selligueain A, a novel highly sweet proanthocyanidin from the rhizomes of Selliguea feei. J Nat Prod 56(9):1532–1538
Birch GG (1987) Sweetness and sweeteners. Endeavour 11(1):21–24
Birch G, Mylvaganam A (1976) Evidence for the proximity of sweet and bitter receptor sites. Nature 260:632–634
van der Heijden A, van der Wel H, Peer HG (1985) Structure-activity relationships in sweeteners. I. Nitroanilines, sulphamates, oximes, isocoumarins and dipeptides. Chem Senses 10(1):57–72
Katritzky AR, Petrukhin R, Perumal S, Karelson M, Prakash I, Desai N (2002) A QSPR study of sweetness potency using the CODESSA program. Croat Chem Acta 75(2):475–502
Acknowledgments
Cristian Rojas is grateful for his PhD Fellowship from the National Secretary of Higher Education, Science, Technology and Innovation (SENESCYT) from the Republic of Ecuador, as well as for the financial support provided by the Ministry of Foreign Affairs and International Cooperation (FARNESINA) from the Italian Government for the PhD research conducted at the University of Milano-Bicocca.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published as part of the special collection of articles “CHITEL 2015 - Torino - Italy”.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rojas, C., Ballabio, D., Consonni, V. et al. Quantitative structure–activity relationships to predict sweet and non-sweet tastes. Theor Chem Acc 135, 66 (2016). https://doi.org/10.1007/s00214-016-1812-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-016-1812-1