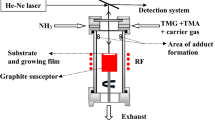
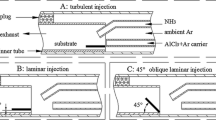
Abstract
This study presents numerical modeling based on a relatively limited number of gas-phase and surface reactions to simulate the growth rate of aluminum nitride layers on AlN templates and c-plane sapphire in a broad range of deposition parameters. Modeling results have been used to design particular experiments in order to understand the influence of the process parameters on the crystal quality of AlN layers grown in a high-temperature hydride vapor-phase epitaxy process fed with NH3, AlCl3, and H2. Modeling results allow to access to very interesting local quantities such as the surface site ratio and local supersaturation. The developed universal model starting from local parameters might be easily transferred to other reactor geometry and process conditions. Among the investigated parameters (growth rate, temperature, local supersaturation, gas-phase N/Al ratio, and local surface site N/Al ratio), only the growth rate/supersaturation or growth rate/temperature relationships exhibit a clear process window to use in order to succeed in growing epitaxial AlN layers on c-plane sapphire or AlN templates. Gas-phase N/Al ratio and local surface site N/Al ratio seem to play only a secondary role in AlN epitaxial growth.









Similar content being viewed by others
Abbreviations
- (S) :
-
Surface site
- (B) :
-
Bulk or solid species
- [A] :
-
Gas-phase concentration of species A (mol m−3)
- a k :
-
Pre-exponential factor for reaction k (consistent units)
- [C(S)]:
-
Surface site concentration of species C (mol m−2)
- E ak :
-
Activation energy for reaction k (J mol−1)
- k k :
-
Rate constant for reaction k (s−1 or mol−1 m3 s or mol−2 m6 s)
- M :
-
Unspecified species (–)
- M A :
-
Molar mass for gaseous specie A (kg mol−1)
- P A :
-
Partial pressure of A at the growing AlN surface (Pa)
- \(P_{A}^{*}\) :
-
Equilibrium pressure of A versus AlN in vacuum (Pa)
- R :
-
Ideal gas constant (J mol−1 K−1)
- T :
-
Temperature (K)
- β k :
-
Temperature exponent for reaction k (–)
- γ A :
-
Sticking coefficient for gaseous species A (–)
- γ 0 :
-
Pre-exponential factor for the sticking coefficient (–)
- Γ tot :
-
Total surface site concentration (mol m−2)
- v k :
-
Production rate of solid surface for reaction k (mol m−2 s−1)
References
Kinoshita T, Hironaka K, Obata T, Nagashima T, Dalmau R, Schlesser R, Moody B, Xie JQ, Inoue S, Kumagai Y, Koukitu A, Sitar Z (2012) Appl Phys Express 5:122101
Ballandras S, Reinhardt A, Laude V, Soufyane A, Camou S, Daniau W, Pastureaud T, Steichen W, Lardat R, Solal M, Ventura P (2004) J Appl Phys 96:7731–7741
Piazza G, Felmetsger V, Muralt P, Olsson RH, Ruby R (2012) MRS Bull 37:1051–1061
Slack GA, McNelly TF (1977) J Cryst Growth 42:560–563
Tian W, Yan WY, Dai JN, Li SL, Tian Y, Hui X, Zhang JB, Fang YY, Wu ZH, Chen CQ (2013) J Phys D Appl Phys 46:065303
Nickel KG, Riedel R, Petzow G (1989) J Am Ceram Soc 72(10):1804–1810
Kumagai Y, Yamane T, Miyaji T, Murakami H, Kangawa Y, Koukitu A (2003) Phys Status Solidi (c) 7:2498–2501
Kumagai Y, Yamane T, Koukitu A (2005) J Cryst Growth 281:62–67
Freitas JA, Culbertson JC, Mastro MA, Kumagai Y, Koukitu A (2012) J Cryst Growth 350:33–37
Nagashima T, Harada M, Yanagi H, Kumagai Y, Koukitu A, Takada K (2007) J Cryst Growth 300:42–44
Eriguchi K, Murakami H, Panyukova U, Kumagai Y, Ohira S, Koukitu A (2007) J Cryst Growth 298:332–335
Eriguchi K, Hiratsuka T, Marukami H, Kumagai Y, Koukitu A (2008) J Cryst Growth 310:4016–4019
Tajima J, Murakami H, Kumagai Y, Takada K, Koukitu A (2009) J Cryst Growth 311:2837–2839
Claudel A, Blanquet E, Chaussende D, Audier M, Pique D, Pons M (2009) J Cryst Growth 311:3371–3379
Balaji M, Claudel A, Fellmann V, Gelard I, Blanquet E, Boichot R, Pierret A, Attal-Tretout B, Crisci A, Coindeau S, Roussel H, Pique D, Baskar K, Pons M, Alloy J (2012) Compound 526:103–109
Claudel A, Blanquet E, Chaussende D, Boichot R, Doisneau B, Berthome G, Crisci A, Mank H, Moisson C, Pique D, Pons M (2011) J Cryst Growth 335:17–24
Allendorf MD, Osterheld TH In: Proceedings of the electrochemical society, vol 96-5
Mc Daniel AH, Allendorf MD (1998) J Phys Chem A 102:7804–7812
McDaniel AH, Allendorf MD (1997) In: Proceedings of the CVDXIV/EUROCVD 11, vol 97-25. The Electrochemical Society, Pennington, p 40
Allendorf MD, Melius CF, Osterheld TH (1996) Mater Res Soc Proc 410:459
Miller JA, Bowman CT (1989) Prog Energy Combust Sci 15:287
Dollet A, Casaux Y, Chaix G, Dupuy C (2002) Thin Solid Films 406:1–16
Swihart MT, Catoire L, Legrand B, Gökalp I, Paillard C (2003) Combust Flame 132:91–101
Cai D, Zheng LL, Zhang H, Tassev VL, Bliss VF (2005) J Cryst Growth 276:182–193
Cai D, Zheng LL, Zhang H, Tassev VL, Bliss VF (2006) J Cryst Growth 293:136–145
Segal AS, Bazarevskiy DS, Bogdanov MV, Yakovlev EV (2009) Phys Status Solidi (c) 6:S329–S332
Boichot R, Claudel A, Baccar N, Milet A, Blanquet E, Pons M (2010) Surf Coat Technol 205:1294
Kee RJ, Rupley FM, Meeks E, Miller JA Chemkin-III: a FORTRAN chemical kinetics package for the analysis of gas phase chemical and plasma kinetics. Sandia National Laboratories. Livermore, California
Bird RB, Stewart WE, Lighfoot EN (2002) Transport phenomena, 2nd edn. Wiley, New York
Svehla RA (1962) NASA technical report R-132. NASA-TR-R-132
Taylor R, Krishna R (1993) Multicomponent mass transfer. Wiley, New York
JANAF Thermochemical tables, 3rd edn. The American Chemical Society (Washington) and the American Institute of Physics (New York) for the National Bureau of Standards, USA
Coltrin ME, Kee RJ, Rupley FM (1990) Surface CHEMKIN. Sandia National Laboratories. Livermore, California
Blocher JM (1974) J Vac Sci Technol 11(4):680–686
Räback P, Nieminen R, Yakimova R, Tuominen M, Janzén E (1997) J Electrochem Soc 144:1024–1027
Bloem J, Oei YS, de Moor HHC, Hansen JHL, Giling LJ (1985) J Electron Soc 132(8):1973–1980
Hwang NM, Yoon DY (1994) J Mater Sci Lett 13(19):1437–1439
Giling LJ, De Moor HHC, Jacobs WPJH, Saaman AA (1986) J Cryst Growth 78(2):303–321
Tai C, Shih C-Y (1996) J Cryst Growth 160(1–2):186–189
Vahlas C, Hwang NM, Gueroudji L, Maury F (1998) Chem Vap Depos 4(3):96–99
Boichot R, Coudurier N, Mercier F, Lay S, Crisci A, Coindeau S, Claudel A, Blanquet E, Pons M (2013) Surf Coat Technol. doi:10.1016/j.surfcoat.2013.08.016
Pons M, Boichot R, Coudurier N, Claudel A, Blanquet E, Lay S, Mercier F, Pique D (2013) Surf Coat Technol 230:111–118
Claudel A, Blanquet E, Chaussende D, Boichot R, Martin R, Mank H, Crisci A, Doisneau B, Chaudouet P, Coindeau S, Pique D, Pons M (2011) J Electrochem Soc 158(3):H328–H332
Kumagai Y, Enatsu Y, Ishizuki M, Kubota Y, Tajima J, Nagashima T, Murakami H, Takada K, Koukitu A (2010) J Cryst Growth 312:2530–2536
Claudel A, Blanquet E, Chaussende D, Boichot R, Doisneau B, Berthomé G, Crisci A, Mank H, Moisson C, Pique D, Pons M (2011) J Cryst Growth 335(1):17–24
Akiyama K, Araki T, Murakami H, Kumagai Y, Koukitu A (2007) Phys Status Solidi (c) 4(7):2297–2300
Nagashima T, Harada M, Yanagi H, Fukuyama H, Kumagai Y, Koukitu A, Takada K (2007) J Cryst Growth 305:355–359
Author information
Authors and Affiliations
Corresponding author
Additional information
Published as part of a special collection of articles focusing on chemical vapor deposition and atomic layer deposition.
Rights and permissions
About this article
Cite this article
Boichot, R., Coudurier, N., Mercier, F. et al. CFD modeling of the high-temperature HVPE growth of aluminum nitride layers on c-plane sapphire: from theoretical chemistry to process evaluation. Theor Chem Acc 133, 1419 (2014). https://doi.org/10.1007/s00214-013-1419-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-013-1419-8