Abstract
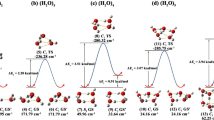
What would be the effects on the nature of hydrogen bonds, on the energies, and on the overall structural possibilities of replacing some hydrogen atoms by small hydrophobic groups in small water networks? Aiming at investigating this question, we performed an exhaustive search of the conformational space of the (Methanol)2(Water)3 representative model system, characterized the results, and made key comparative analysis with pentameric pure water clusters. The potential energy surface yielded a global minimum structural motif consisting of several puckered ring-like cyclic isomers very close in energy to each other. They are followed by other structural motifs, which, contrary to conventional belief, would also contribute to the properties of a macroscopic sample of this composition. We found that the C–H···O interactions play a subordinate structural role and preferably accommodate to the established O–H···O based structures. In comparison with the pure (H2O)5 case, we showed that (1) the same basic structural motifs and in a similar hierarchy energy order are obtained, but with a richer structural isomerism; (2) in general, the bonding is reinforced by the increase in the electrostatic and in the “degree of covalency” of the hydrogen-bonding components. Therefore, at least for this small cluster size, methyl groups slightly affect the structural isomerism and reinforce the hydrogen bonding. Additionally, we identified general factors of instability of the more unstable structures.





Similar content being viewed by others
References
Xantheas SS (2010) Recent theoretical and experimental advances in hydrogen bonded clusters. Nato Science Series: C Mathematical and Physical Sciences, Volume 561 Kluwer Academic Publishers, Dordrecht, The Netherlands
Schuster P, Wolschann P (1999) In: Schuster P, Wolschann P (eds) Hydrogen bonding: from small clusters to biopolymers. Springer, Wien
Guo JH, Luo Y, Augustsson A, Kashtanov S, Rubensson JE, Shuh DK, Agren H, Nordgren J (2003) Phys Rev Lett 91:157401–157402
Ruckenstein E, Shulgin IL, Tilson L (2005) J Phys Chem A 109:807
Teschke O, de Souza EF (2005) Chem Phys Lett 403:95
Teschke O, de Souza EF (2005) Phys Chem Chem Phys 7:3856
Perera A, Mazighi R, Kežíc BJ (2012) Chem Phys 136:174516
Bagchi B (2012) Chem Phys Lett 9:1
Dougherty RC, Howard LN (1998) J Chem Phys 109:7379
Lin K, Zhou X, Luo Y, Liu S (2010) J Phys Chem B 114:3567
Tamenori Y, Okada K, Takahashi O, Arakawa S, Tabayashi K, Hiraya A, Gejo T, Honma K (2008) J Chem Phys 128:124321
Kumagai T (2012) Visualization of hydrogen-bond dynamics: water-based model systems on a Cu(110) surface. Springer, Japan
Marechal Y (2007) The hydrogen bond and the water molecule: the physics and chemistry of water. Aqueous and Bio-Media, Elsevier
Eisenberg D, Kauzmann W (2005) The structure and properties of water. Oxford University Press, Oxford
Xantheas SS (2000) Chem Phys 258:225
Goldman N, Fellers RS, Brown MG, Braly LB, Keoshian CJ, Leforestier C, Saykally RJ (2002) J Chem Phys 116:10148
Nielsen IMB, Seidl ET, Janssen CL (1999) J Chem Phys 110:9435
Ren P, Ponder JW (2005) J Phys Chem B 107:5933
Hincapié G, Acelas N, Castaño M, David J, Restrepo A (2010) J Phys Chem A 114:7809
Han G, Ding Y, Qian P, Zhang C, Song W (2012) Int J Quantum Chem. doi:10.1002/qua.24352
Pérez J, Hadad C, Restrepo A (2008) Int J Quantum Chem 108:1653
Ramirez F, Hadad CZ, Guerra D, David J, Restrepo A (2011) Chem Phys Lett 507:229
Mandal A, Prakash M, Kumar RM, Parthasarathi R, Subramanian V (2010) J Phys Chem A 114:2250
Stockman PA, Blake GA, Lovas FJ, Suenram RD (1997) J Chem Phys 107:3782
Iosue JL, Benoit DM, Clary DC (1999) Chem Phys Lett 301:272
Jursic BS (1999) J Mol Struct Theochem 466:203
Gonzáles L, Mó O, Yáñez M (1998) J Chem Phys 109:139
Mejía SM, Espinal JF, Restrepo A, Mondragón F (2007) J Phys Chem A 111:8250
Mejía SM, Espinal JF, Mondragón F (2009) J Mol Struct Theochem 901:186
Mejía SM, Flórez E, Mondragón F (2012) J Chem Phys 136:144306
Raina G, Kulkarni GU (2001) Chem Phys Lett 337:269
Tsuneishi S (2011) Import Tuner magazine, January
Snyder LR, Kirkland JJ, Dolan JW (2009) Introduction to modern liquid chromatography. Wiley, New York
Pérez J, Restrepo A (2008) ASCEC V-02: Annealing Simulado con Energía Cuántica. Property, Development and Implementation: Grupo de Química–Física Teórica, Instituto de Química, Universidad de Antioquia: Medellín, Colombia
David J, Guerra D, Restrepo A (2009) J Phys Chem A 113:10167
Murillo J, David J, Restrepo A (2010) Phys Chem Chem Phys 12:10963
Liedl K, Sekušak S, Mayer E (1997) J Am Chem Soc 119:3782
Peterson K, Dunning P (1995) J Chem Phys 102:2032
Xantheas S (1996) J Chem Phys 104:8821
Feyereisen M, Dixon D (1996) J Phys Chem 100:2993
Frisch MJ et al (2004) GAUSSIAN 03, Revision E.01, Gaussian, Inc., Wallingford CT
AIMAll (Version 10.09.12), T A Keith (2010) (aim.tkgristmill.com)
Bader R (1994) Atoms in molecules: a quantum theory. Oxford University Press, USA
Gillespie RJ, Hargittai I (2012) (1996) The VSEPR model of molecular geometry. Dover Books on Chemistry, United States
Hoffmann R, von Ragué Schleyer P, Schaefer HF (2008) Angew Chem Int Edit 47:7164
Su P, Li H (2009) J Chem Phys 131:014102
Kitaura K, Morokuma K (1976) Int J Quantum Chem 10:325
Grabowski S (2011) J Chem Rev 111:2597
Shaik SS, Hiberty PC (2008) A chemist’s guide to valence bond theory. Wiley-Interscience, New Jersey
Cotton FA (1990) Chemical applications of group theory. John Wiley & Sons, New York
Parthasarathi R, Elango M, Subramanian V, Sathyamurthy N (2009) J Phys Chem A 113:3744
Espinosa E, Molis E, Lecomte C (1998) Chem Phys Lett 285:170
Jenkins S, Morrison I (2000) Chem Phys Lett 317:97
Oliveira BG, Vasconcellos MLAA (2006) J Mol Struct Theochem 774:83
Bondi A (1964) J Phys Chem 68:441
Koch U, Popelier PLA (1995) J Phys Chem 99:9747
Acknowledgments
C.Z.H. and A. R. are grateful to Universidad de Antioquia for partial financial support through Estrategia de Sostenibilidad 2013-2014 project, and C.Z.H. thanks Proyecto CIEN-CODI IN10184CE. S.J gratefully acknowledges the support of the One Hundred Talents of Hunan program for support and the aid program for Science and Technology Innovative Research Team in Higher Educational Institutions of Hunan Province. The National Natural Science Foundation of China is also gratefully acknowledged, project approval number: 21273069.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hadad, C.Z., Restrepo, A., Jenkins, S. et al. Hydrophobic meddling in small water clusters. Theor Chem Acc 132, 1376 (2013). https://doi.org/10.1007/s00214-013-1376-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-013-1376-2