Abstract
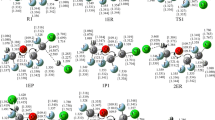
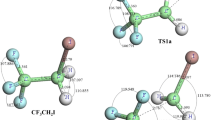
This paper presents the theoretical studies of the reactions of Cl atoms with CF3CH2OCH3, CF3CH2OCH2F and CF3CH2OCHF2 using an ab initio direct dynamics theory. The geometries and vibrational frequencies of the reactants, complexes, transition states and products are calculated at the MP2/6-31+(d,p) level. The minimum energy path is also calculated at same level. The MC-QCISD method is carried out for further refining the energetic information. The rate constants are evaluated with the canonical variational transition state theory (CVT) and CVT with small curvature tunneling contributions in the temperature range 200–1,500 K. The results are in good agreement with experimental values.









Similar content being viewed by others
References
World Meteorological Organization (WMO) (1994) Scientific assessment of ozone depletion. Report No. 37, Geneva, WMO
Wallington TJ, Schneider WF, Sehested J, Bilde M, Platz J, Nielsen OJ, Christensen LK, Molina MJ, Molina LT, Wooldridge PWJ (1997) Phys Chem A. 101:8264
DeMore WB, Sander SP, Golden DM, Hampson RF, Kurylo MJ, Howard CJ, Ravishankara AR, Kolb CE, Molina MJ (1997) Chemical kinetics and photochemical data for use in stratospheric. JPL Publication 97:4
Oyaro N, Sellevag SR, Nielsen CJJ (2005) Phys Chem A 109:337
Kyriakos GK, Yannis GL, Panos PJ (1998) Phys Chem. A 102:8620–8625
Beach SD, Hickson KM, Smith IWM, Tuckett RP (2001) Phys Chem Chem Phys 3:3064
Wallington TJ, Hurley MD, Fedotov V, Morrell C, Hancock GJ (2002) Phys Chem A 106:8391
Hickson KM, Smith IWM (2001) Int J Chem Kinet 33:165
Yang L, Liu JY, Wang L, He HQ, Wang Y, Li ZSJ (2008) Comput Chem 29:550–561
Truhlar DG (1995) In: Heidrich D (ed) The reaction path in chemistry: current approaches and perspectives. Kluwer, Dordrecht, p 229
Truhlar DG, Garrett BC, Klippenstein SJJ (1996) Phys Chem 100:12771
Hu WP, Truhlar DGJ (1996) Am Chem Soc 118:860
Corchado JC, Chuang YY, Fast PL, Villa J, Hu WP, Liu YP, Lynch GC, Nguyen KA, Jackels CF, Melissas VS, Lynch BJ, Rossi I, Coitino EL, Ramos AF, Pu J, Albu TV (2002) POLYRATE version 9.1. Department of Chemistry and Supercomputer Institute, University of Minnesota, Minneapolis
Truhlar DG, Isaacson AD, Garrett BC (1985) In: Baer M (ed) The theory of chemical reaction dynamics, vol 4. CRC Press, Boca Raton, p 65
Truhlar DG, Garrett BC (1980) Acc Chem Res 13:440
Duncan WT, Truong TNJ (1995) Chem Phys 103:9642
Frisch MJ, Head-Gordon M, Pople JA (1990) Chem Phys Lett 166:275
Head-Gordon M, Pople JA, Frisch MJ (1988) Chem Phys Lett 153:503
Zhang H, Liu JY, Li ZS, Sheng L, Wu JY, Sun CC (2005) Chem Phys Lett 405:240–245
Yu X, Li SM, Xu ZF, Li ZS, Sun CCJ (2001) Phys. Chem. A 105:7072–7078
Zhang QZ, Wang SK, Gu YSJ (2002) Phys Chem. A 106:3796–3803
Li QS, Luo Q (2003) J Phys Chem. A 107:10435–10440
Yu YM, Feng SY, Feng DC (2005) J Phys Chem. A 109:3663–3668
Zhang QZ, Gu YS, Wang SKJ (2003) Phys Chem. A 107:8295–8301
Fast PL, Truhlar DGJ (2000) Phys Chem A 104:6111
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JAJ, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) GAUSSIAN03 program package. Pittsburgh, Gaussian
Lu DH, Truong TN, Melissas VS, Lynch GC, Liu YP, Grarrett BC, Steckler R, Issacson AD, Rai SN, Hancock GC, Lauderdale JG, Joseph T, Truhlar DG (1992) Comput Phys Commun 71:235
Garrett BC, Truhlar DG, Grev RS, Magnuson AWJ (1980) Phys Chem 84:1730
Liu Y-P, Lynch GC, Truong TN, Lu D-H, Truhlar DG, Garrett BCJ (1993) Am Chem Soc 115:2408
Truhlar DGJ (1991) Comput Chem 12:266
Chuang YY, Truhlar DGJ (2000) Chem Phys 112:1221
Rayez MT, Rayez JCJ (1994) Phys Chem 98:11342
Harmony MD, Laurie VW, Ramsay RL, Lovas FJ, Lafferty WJ, Maki AGJ (1979) Phys Chem 8:619
Shimanouchi T (1972) Tables of molecular vibrational frequencies consolidated, vol I. National Bureau of Standards, US GPO, Washington, DC, pp 1–160
Shimanouchi T (1972) Tables of molecular vibrational frequencies consolidated, volume II. J Phys Chem Ref Data 6(3):993–1102
Chase MW, Davies CA (1985) Janaf thermochemical tables, 3rd edn. Ref Data, 14,1
Acknowledgments
The authors thank Professor Donald G. Truhlar for providing POLYRATE 9.1 program. This work is supported by the National Natural Science Foundation of China (50743013, 20973049), the Foundation for University Key Teacher by the Department of Education of Heilongjiang Province (1152G010), the SF for leading experts in academe of Harbin of China (2007RFXXG027), the SF for Postdoctoral of Heilongjiang province of China (LBH-Q07058), and Natural Science Foundation of Heilongjiang Province (B200605), The Foundation of Graduate Innovation of the Education Department of Heilongjiang province (YJSCX2009-055HLJ).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
214_2010_746_MOESM1_ESM.docx
Supporting Information Available: Table S1 Calculated frequencies (cm−1) for the transitions states at the MP2/6-31+G(d,p) level. Table S2 Calculated and experimental frequencies (cm−1) for the reactants, products and complexes at the MP2/6-31+G(d,p) level. Table S3 Relative energies of the stationary points in terms of enthalpy and Gibbs free energy (Hartree) calculated at the MP2/6-31+G(d,p) level. Table S4 The TST, CVT, ZCT and SCT rate constants calculated at the MC-QCISD//MP2/6-31+G(d,p) level for three reactions, R1, R2, and R3, between 200 and 1,500 K (cm3 molecule−1 s−1). Fig. S1 Optimized geometries of the reactants, complexes and products at the MP2/6-31+G(d,p) level. (DOCX 1359 kb)
Rights and permissions
About this article
Cite this article
Zhang, H., Liu, Cy., Zhang, Gl. et al. Theoretical studies of the reactions of Cl atoms with CF3CH2OCH n F(3−n) (n = 1, 2, 3). Theor Chem Acc 127, 551–560 (2010). https://doi.org/10.1007/s00214-010-0746-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00214-010-0746-2