Abstract
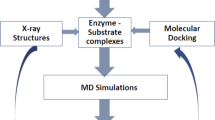
Glycosidases constitute a vast family of enzymes that catalyze the breaking and formation of glycosidic bonds. The synthesized oligosaccharides, being crucial to life, are involved in many biochemical processes, particularly in the pharmaceutical and food industries. The proposed catalytic mechanism of retaining glycoside hydrolases (glycosidases) occurs via a double displacement mechanism involving a covalent glycosyl enzyme intermediate. During the transglycosylation reactions, the control of the stereoselectivity for the formation of the new bond remains a complicated problem in the chemical synthesis of oligosaccharides. In this paper, docking and molecular dynamics methods were used to study the second step of the mechanism of transglycosylation in retaining glycosidases from six microorganisms with known stereoselectivity. Using the natural substrates as donor and acceptor molecules, we were able to corroborate and provide structural information about the active site, the trapped monosaccharide acceptor and the bound intermediates during the step that precedes transglycosylation, as well as identify and understand the commonly displayed stereoselectivity by these glycosidases in nature. The information obtained with this procedure helps to recognize, explain and predict the stereoselectivity of the sugars studied. These kind of procedures can be used to improve the efficiency of large-scale industrial synthesis of a specific sugar.




Similar content being viewed by others
References
Perugino G, Trincone A, Rossi M, Moracci M (2004) Trends Biotechnol 22:31–37. doi:10.1016/j.tibtech.2003.10.008
Maugard T, Gaunt D, Legoy MD, Besson T (2003) Biotechnol Lett 25:623–629. doi:10.1023/A:1023060030558
Cruz-Guerrero AE, Gomez-Ruiz L, Viniegra-Gonzalez G, Barzana E, Garcia-Garibay M (2006) Biotechnol Bioeng 93:1123–1129. doi:10.1002/bit.20824
Holzapfel WH, Schillinger U (2002) Food Res Intern 35:109–116. doi:10.1016/S0963-9969(01)00171-5
Jakeman DL, Withers SG (2002) Trends Glycosci Glycotechnol 14(75):13–25
Ajisaka K, Yamamoto Y (2002) Trends Glycosci Glycotechnol 14(75):1–11
Reuter S, Nygaard AR, Zimmermann W (1999) Enzyme Microb Technol 25:509–516. doi:10.1016/S0141-0229(99)00074-5
Zechel DL, Withers SG (2000) Acc Chem Res 33:11–18. doi:10.1021/ar970172
Koshland DE (1953) Biol Rev Camb Philos Soc 28:416–436. doi:10.1111/j.1469-185X.1953.tb01386.x
Jahn M, Withers SG (2003) Biocatal Biotransformation 21:159–166. doi:10.1080/1024220310001614351
Crout DHG, Vic G (1998) Curr Opin Chem Biol 2:98–111. doi:10.1016/S1367-5931(98)80041-0
Mayer C, Jakeman DL, Mah M, Karjala G, Gal L, Warren RAJ, Withers SG (2001) Chem Biol 8:437–443. doi:10.1016/S1074-5521(01)00022-9
Gu QM (1999) J Environ Polym Degrad 7:1–7. doi:10.1023/A:1021833917049
Yoon JH, Ajisaka K (1996) Carbohydr Res 292:153–163
Zinin AI, Eneyskaya EV, Shabalin KA, Kulminskaya AA, Shishlyannikov SM, Neustroev KN (2002) Carbohydr Res 337:635–642. doi:10.1016/S0008-6215(02)00027-7
Zeng X, Murata T, Ajisaka K, Usui T (2000) Carbohydr Res 325:120–131. doi:10.1016/S0008-6215(99)00303-1
Bernstein FCKT, Williams GJB, Meyer EF, Brice MD, Rodgers JR, Kennard O, Shimanouchi T, Tasumi M (1977) J Mol Biol 112(3):535–542. doi:10.1016/S0022-2836(77)80200-3
Accelrys (1993) InsightII v.2.3.0. San Diego, CA
Brás NF, Moura-Tamames SA, Fernandes PA, Ramos MJ (2008) J Comput Chem 29(15):2565–2574
Jones G, Willett P, Glen RC, Leach AR, Taylor R (1997) J Mol Biol 267:727–748. doi:10.1006/jmbi.1996.0897
Verdonk ML, Cole JC, Hartshorn MJ, Murray CW, Taylor RD (2003) Proteins Struct Funct Genet 52:609–623. doi:10.1002/prot.10465
Case DA, Cheatham TE, Darden TI, Gohlke H, Luo R, Merz KM, Onufriev A, Simmerling C, Wang B, Woods R (2005) J Comput Chem 26:1668
Kirschner KN, Woods RJ (2001) Proc Natl Acad Sci USA 98:10541–10545. doi:10.1073/pnas.191362798
Kirschner KN, Woods RJ (2001) J Phys Chem A 105:4150–4155. doi:10.1021/jp004413y
Basma M, Sundara S, Calgan D, Vernali T, Woods RJ (2001) J Comput Chem 22:1125–1137. doi:10.1002/jcc.1072
Asensio JL, Jimenez Barbero J (1995) Biopolymers 35:55–73. doi:10.1002/bip.360350107
Izaguirre JA, Catarello DP, Wozniak JM, Skeel RD (2001) J Chem Phys 114:2090–2098. doi:10.1063/1.1332996
Loncharich RJ, Brooks BR, Pastor RW (1992) Biopolymers 32:523–535. doi:10.1002/bip.360320508
Cornell WD, Cieplak P, Bayly CI, Gould IR, Merz KM, Ferguson DM, Spellmeyer DC, Fox T, Caldwell JW, Kollman PA (1995) J Am Chem Soc 117:5179–5197. doi:10.1021/ja00124a002
Hammonds KD, Ryckaert JP (1991) Comput Phys Commun 62:336–351. doi:10.1016/0010-4655(91)90105-T
Drone J, Feng HY, Tellier C, Hoffmann L, Tran V, Rabiller C, Dion M (2005) Eur J Org Chem 1977–1983. doi:10.1002/ejoc.200500014
Huo S, Massova I, Kollman PA (2002) J Comput Chem 23:15. doi:10.1002/jcc.1153
Rocchia W, Sridharan S, Nicholls A, Alexov E, Chiabrera A, Honig B (2002) J Comput Chem 23:128. doi:10.1002/jcc.1161
Rocchia W, Alexov E, Honig B (2001) J Phys Chem B 105:6507. doi:10.1021/jp010454y
Juers DH, Heightman TD, Vasella A, McCarter JD, Mackenzie L, Withers SG, Matthews BW (2001) Biochemistry 40:14781–14794. doi:10.1021/bi011727i
Juers DH, Jacobson RH, Wigley D, Zhang XJ, Huber RE, Tronrud DE, Matthews BW (2000) Protein Sci 9:1685–1699
Rojas AL, Nagem RAP, Neustroev KN, Arand M, Adamska M, Eneyskaya EV, Kulminskaya AA, Garratt RC, Golubev AM, Polikarpov I (2004) J Mol Biol 343:1281–1292. doi:10.1016/j.jmb.2004.09.012
Gloster TM, Roberts S, Ducros VMA, Perugino G, Rossi M, Hoos R, Moracci M, Vasella A, Davies GJ (2004) Biochemistry 43:6101–6109. doi:10.1021/bi049666m
Mackenzie LF, Sulzenbacher G, Divne C, Jones TA, Woldike HF, Schulein M, Withers SG, Davies GJ (1998) Biochem J 335:409–416
Ducros VMA, Tarling CA, Zechel DL, Brzozowski AM, Frandsen TP, von Ossowski I, Schulein M, Withers SG, Davies GJ (2003) Chem Biol 10:619–628. doi:10.1016/S1074-5521(03)00143-1
Cutfield JFSPA, Cutfield SM (2000) Protein Eng 13:735–738. doi:10.1093/protein/13.10.735
Stubbs HJ, Brasch DJ, Emerson GW, Sullivan PA (1999) Eur J Biochem 263:889–895. doi:10.1046/j.1432-1327.1999.00581.x
Jahn M, Stoll D, Warren RAJ, Szabó L, Singh P, Gilbert HJ, Ducros VM-A, Davies GJ, Withers G (2003) Chem Commun 12:1327–1329. doi:10.1039/b302380j
Fort S, Boyer V, Greffe L, Davies GJ, Moroz O, Christiansen L, Schulein M, Cottaz S, Driguez H (2000) J Am Chem Soc 122:5429–5437. doi:10.1021/ja9936520
Acknowledgments
The authors would like to thank the Portuguese Science and Technology Foundation (FCT-MCTES) for financial support (scholarship SFRH/BD/31359/2006).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Brás, N.F., Fernandes, P.A. & Ramos, M.J. Docking and molecular dynamics studies on the stereoselectivity in the enzymatic synthesis of carbohydrates. Theor Chem Account 122, 283–296 (2009). https://doi.org/10.1007/s00214-009-0507-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00214-009-0507-2