Abstract
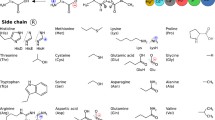
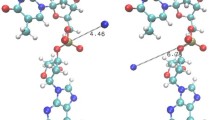
Association between NO and each of the 20 amino acids and their related organic functional groups was studied by exploring the configuration space of the potential energy of association surface by using the multiple minima hypersurface procedure. AM1 semiempirical Hamiltonian was used in order to explore such complex hypersurfaces of biological molecular interactions at finite computational times. An appropriate test with a set of NO and small molecule complexes obtained at the MP2/6-311++g(2d,2p) level of theory was also carried out. Stabilization energies of larger models were also evaluated at the conventional PBE1PBE/6-31g(d,p) DFT level. NO–aminoacid hypersurface explorations yielded that interactions of NO with NH group together with the C=O belonging to the backbone appeared predominant in all cases. Models of polar aminoacids and NO also show stable interactions with the lateral chains. Interactions with charged amino acids were found as the most stable and Lys was, undoubtedly, the preferred association. The study of these kinds of interactions must take into account the deepest and other minima because the entropy of association plays an important role.
Similar content being viewed by others

References
Moncada S and Higgs A (1993). New Engl J Med 329: 2002
Koshland DE Jr (1992). Science 258: 1861
Richter-Addo GB, Legzdinns P and Burstyn J (2002). Chem Rev 102: 358
Radi R (1996). Chem Res Toxicol 9: 828
Scherlis DA, Martí MA, Ordejón P and Estrín D (2002). Int J Quantum Chem 90: 1505
Bossa C, Anselmi M, Roccatano D, Amadei A, Vallone B, Brunori M and Di Nola A (2004). Biophys J 86: 3855
Cohen J, Arkhipov A, Braun R and Schulten K (2006). Biophys J 91: 1844
Fraga S and Parker JMR (1994). Amino Acids 7: 175
Duijneveldt FB, Duijneveldt-van de Rijdt JGCM and Lenthe JH (1994). Chem Rev 94: 873
Hadži D (ed.) (1997) Theoretical treatments of hydrogen bonding. Wiley, New York, Chapter 5, p 95
Montero LA, Esteva AM, Molina J, Zapardiel A, Hernández L, Márquez H and Acosta A (1998). J Am Chem Soc 120: 12023
Montero LA, Molina J and Fabian J (2000). Int J Quantum Chem 79: 8
Crespo-Otero R, Montero LA; Stohrer W-D and García de la Vega JM (2005). J Chem Phys 123: 134107
Boys SF and Bemardi F (1970). Mol Phys 9: 553
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, and Pople JA (2004). Gaussian 03, Revision c.02. Gaussian, Inc., Wallingford
Codorniu-Hernández E, Mesa-Ibirico A, Montero-Cabrera LA, Martínez-Luzardo F, Borrmann T and Stohrer W-D (2002). THEOCHEM 623: 63
http://karin.qct.fq.oc.uh.cu/mmh/. Available by request
George L, Sanchez-Garcia E and Sander W (2004). J Phys Chem A 107: 6850
Sanchez-Garcia E, George L, Montero LA and Sander W (2004). J Phys Chem A 108: 11846
Codorniu-Hernández E, Mesa-Ibirico A, Hernández -Santiesteban R, Montero-Cabrera LA, Santana-Romero JL, Borrmann T, Stohrer W-D and Martínez-Luzardo (2005). Int J Quantum Chem 3: 82
Padrón-García JA, Crespo-Otero R, Hernández-Rodríguez EW, Garriga P, Montero LA and García-Piñeiro JC (2004). Proteins 57: 392
Schönfeld P, Fabian J and Montero L (2005). Biophys J 89: 1504
Stewart JJP (1993–1997) MOPAC, v. 6, Release for PC computers in the laboratory of computational and theoretical chemistry, Universidad de La Habana
Farrugia LJ (1997). J Appl Crystallogr 30: 565
Zhao Y and Truhlar DG (2006). J Chem Theory Comput 2: 1009
Myszkiewicz G and Sadlej J (2000). Chem Phys Lett 318: 232
Ball DW (1997). J Phys Chem A 101: 4835
Frendin L (1973). Chem Scr 4: 97
Ascenzi P, Colasanti M, Persichini T, Muolo M, Polticelli F, Venturini G, Bordo D and Bolognesi M (2000). Biol Chem 381: 623
Moreno E and León K (2002). Proteins 47: 1
Zhou Z, Todd BD, Travis KP and Sadus RJ (2005). J Chem Phys 123: 054505
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Prof. Serafín Fraga, an unforgettable friend.
Contribution to the Serafin Fraja Memorial Issue.
Rights and permissions
About this article
Cite this article
Crespo-Otero, R., Pérez-Badell, Y., Padrón-García, J.A. et al. Exploring the potential energy surfaces of association of NO with aminoacids and related organic functional groups: the role of entropy of association. Theor Chem Account 118, 649–663 (2007). https://doi.org/10.1007/s00214-007-0346-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00214-007-0346-y