Abstract
Background
Despite growing interest in the (therapeutic) use of intranasal oxytocin administration in children, the potential side-effects of intranasal oxytocin have remained largely unclear to date. The current study is the first double-blind randomized controlled trial to examine side-effects following single administration of oxytocin nasal spray in elementary school-aged children.
Methods
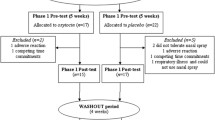
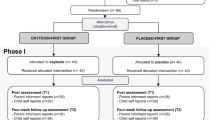
One hundred children (8–12 years old) were randomly assigned to receive oxytocin or placebo nasal spray. We assessed side-effects by means of a standardized, drug-specific questionnaire and an open-ended question at two time points: 90 min after nasal spray administration and 24 h after administration.
Results
There were no significant associations between nasal spray condition and total frequency of reported side-effects or reports of specific side-effects. Children and their mothers were unable to correctly guess nasal spray allocation, further supporting that the subjective experience of oxytocin versus placebo nasal spray effects was similar. Moreover, the majority of reported side-effects were classified as mild and ceased within 24 h after the procedure, indicating that the nasal sprays were well tolerated.
Conclusion
In all, this study is the first randomized controlled trial to provide information on the safety of intranasal oxytocin administration in middle childhood. The current study suggests that single administration of intranasal oxytocin is likely safe in elementary school-aged children.

Similar content being viewed by others
Notes
As computer training condition, in itself or in combination with nasal spray condition, was not significantly associated with total frequency of reported side-effects or incidence of specific side-effects at any time point, we collapsed data from both computer training conditions to assess side-effects of nasal spray condition.
References
Dadds MR, MacDonald E, Cauchi A, Williams K, Levy F, Brennan J (2014) Nasal oxytocin for social deficits in childhood autism: a randomized controlled trial. J Autism Dev Disord 44:521–531. https://doi.org/10.1007/s10803-013-1899-3
DeMayo MM, Song YJC, Hickie IB, Guastella AJ (2017) A review of the safety, efficacy and mechanisms of delivery of nasal oxytocin in children: therapeutic potential for autism and Prader-Willi syndrome, and recommendations for future research. Pediatr Drugs 19:391–410. https://doi.org/10.1007/s40272-017-0248-y
Doig GS, Simpson F (2005) Randomization and allocation concealment: a practical guide for researchers. J Crit Care 20:187–191. https://doi.org/10.1016/j.jcrc.2005.04.005
Einfeld SL, Smith E, McGregor IS, Steinbeck K, Taffe J, Rice LJ, Horstead SK, Rogers N, Hodge MA, Guastella AJ (2014) A double-blind randomized controlled trial of oxytocin nasal spray in Prader Willi syndrome. Am J Med Genet A 164:2232–2239. https://doi.org/10.1002/ajmg.a.36653
Greenhill LL, Vitiello B, Riddle MA et al (2003) Review of safety assessment methods used in pediatric psychopharmacology. J Am Acad Child Psy 42:627–633. https://doi.org/10.1097/01.CHI.0000046841.56865.37
Grossmann K, Grossmann KE, Kindler H, Zimmermann P (2008) A wider view of attachment and exploration: the influence of mothers and fathers on the development of psychological security from infancy to young adulthood. In: Cassidy J, Shaver PR (eds) Handbook of attachment: theory, research, and clinical applications, 2nd edn. The Guilford Press, New York, pp 857–879
Guastella AJ, Hickie IB, McGuinness MM, Otis M, Woods EA, Disinger HM, Chan H, Chen TF, Banati RB (2013) Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrino 38:612–625. https://doi.org/10.1016/j.psyneuen.2012.11.019
Kerns KA, Tomich PL, Kim P (2006) Normative trends in children’s perceptions of availability and utilization of attachment figures in middle childhood. Soc Dev 15:1–22. https://doi.org/10.1111/j.1467-9507.2006.00327.x
Liu JCJ, McErlean RA, Dadds MR (2012) Are we there yet? The clinical potential of intranasal oxytocin in psychiatry. Curr Psychiatr Rev 8:37–48. https://doi.org/10.2174/157340012798994902
Loke YK, Price D, Herxheimer A (2007) Systematic reviews of adverse effects: framework for a structured approach. BMC Med Res Methodol 7:1–9. https://doi.org/10.1186/1471-2288-7-32
MacDonald E, Dadds MR, Brennan JL, Williams K, Levy F, Cauchi AJ (2011) A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research. Psychoneuroendocrino 36:1114–1126. https://doi.org/10.1016/j.psyneuen.2011.02.015
Miller G (2013) The promise and perils of oxytocin. Science 339:267–269. https://doi.org/10.1126/science.339.6117.267
Rief W, Avorn J, Barsky AJ (2006) Medication-attributed adverse effects in placebo groups: implications for assessment of adverse effects. Arch Intern Med 166:155–160. https://doi.org/10.1001/archinte.166.2.155
Van IJzendoorn MH, Bakermans-Kranenburg MJ (2016) The role of oxytocin in parenting and as augmentative pharmacotherapy: critical issues and bold conjectures. J Neuroendocrinol 28. https://doi.org/10.1111/jne.12355
Verhees MWFT, Ceulemans E, Bakermans-Kranenburg MJ, Van IJzendoorn MH, De Winter S, Bosmans G (2017) The effects of cognitive bias modification training and oxytocin administration on trust in maternal support: study protocol for a randomized controlled trial. Trials 18:326. https://doi.org/10.1186/s13063-017-2077-2
Yatawara CJ, Einfeld SL, Hickie IB, Davenport TA, Guastella AJ (2016) The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: a randomized clinical crossover trial. Mol Psychiatry 21:1225–1231. https://doi.org/10.1038/mp.2015.162
Funding
This work was supported by grants G.0774.15 and G.0757.18 from Research Foundation Flanders (FWO) and CREA/12/004 from Research Fund KU Leuven. MJBK was supported by an Advanced Grant from the European Research Council.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Verhees, M.W.F.T., Houben, J., Ceulemans, E. et al. No side-effects of single intranasal oxytocin administration in middle childhood. Psychopharmacology 235, 2471–2477 (2018). https://doi.org/10.1007/s00213-018-4945-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-018-4945-1