Abstract
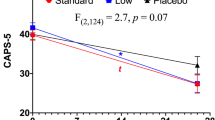
Preclinical and clinical research supports a role for neuroactive steroids in the pathophysiology of posttraumatic stress disorder (PTSD). We investigated ganaxolone (a synthetic 3β-methylated derivative of allopregnanolone, a GABAergic neuroactive steroid) for treatment of PTSD in a proof-of-concept, multisite, double-blind, placebo-controlled trial. Veteran and non-veteran participants (n = 112) were randomized to ganaxolone or placebo at biweekly escalating doses of 200, 400, and 600 mg twice daily for 6 weeks. During an open-label 6-week extension phase, the initial ganaxolone group continued ganaxolone, while the placebo group crossed over to ganaxolone. Eighty-six and 59 participants, respectively, completed the placebo-controlled and open-label phases. A modified intent-to-treat mixed model repeated measures analysis revealed no significant differences between the effects of ganaxolone and placebo on Clinician Administered PTSD Symptom (CAPS) scores, global well-being, negative mood, or sleep. Dropout rates did not differ between groups, and ganaxolone was generally well tolerated. Trough blood levels of ganaxolone at the end of the double-blind phase were, however, lower than the anticipated therapeutic level of ganaxolone in >35% of participants on active drug. Pharmacokinetic profiling of the ganaxolone dose regimen used in the trial and adverse event sensitivity analyses suggest that under-dosing may have contributed to the failure of ganaxolone to out-perform placebo. Future investigations of ganaxolone may benefit from higher dosing, rigorous monitoring of dosing adherence, a longer length of placebo-controlled testing, and targeting of treatment to PTSD subpopulations with demonstrably dysregulated pre-treatment neuroactive steroid levels.
Clinicaltrials.gov identifier: NCT01339689.



Similar content being viewed by others
Notes
Based on the observed general safety of ganaxolone, the upper end of the age range for study eligibility was increased from 55 to 65 years on February 22, 2013, after which an additional 18 participants included in the modified intent-to-treat sample were recruited.
References
American Psychiatric Association (1994) Diagnostic and Statistical Manual of Mental Disorders, 4th edn. Washington, DC, American Psychiatric Association
Bastien CH, Vallières A, Morin CM (2001) Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med 2:297–307. doi:10.1016/S1389-9457(00)00065-4
Bernardy NC, Friedman MJ (2015) Psychopharmacological strategies in the management of posttraumatic stress disorder (PTSD): what have we learned? Curr Psychiatry Rep 17:564. doi:10.1007/s11920-015-0564-2
Bernstein D, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K et al (1994) Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry 151:1132–1136. doi:10.1176/ajp.151.8.1132
Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS (2010) Progress report on new antiepileptic drugs: a summary of the Tenth Eilat Conference (EILAT X). Epilepsy Res 92:89–124. doi:10.1016/j.eplepsyres.2010.09.001
Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS et al (1995) The development of a clinician-administered PTSD scale. J Traum Stress 8:75–90. doi:10.1002/jts.2490080106
Brady K, Pearlstein T, Asnis GM, Baker D, Rothbaum B, Sikes C et al (2000) Efficacy and safety of sertraline treatment of posttraumatic stress disorder. JAMA 283:1837–1844. doi:10.1001/jama.283.14.1837
Breslau N, Davis GC, Peterson EL, Schultz LR (2000) A second look at comorbidity in victims of trauma: the posttraumatic stress disorder-major depression connection. Biol Psychiatry 48:902–909
Chen YF, Yang Y, Hung HJ, Wang SJ (2011) Evaluation of performance of some enrichment designs dealing with high placebo response in psychiatric clinical trials. Contemp Clin Trials 32:592–604
Chhatwal JP, Myers KM, Ressler KJ, Davis M (2005) Regulation of gephyrin and GABAA receptor binding within the amygdala after fear acquisition and extinction. J Neurosci 25:502–506. doi:10.1523/JNEUROSCI.3301-04.2005
Connor KM, Davidson JR (2003) Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC). Dep Anx 18:76–82. doi:10.1002/da.10113
Conybeare D, Behar E, Solomon A, Newman MG, Borkovec TD (2012) The PTSD Checklist-Civilian Version: reliability, validity, and factor structure in a nonclinical sample. J Clin Psychol 68:699–713. doi:10.1002/jclp.21845
Davidson JRT, Rothbaum BO, van der Kolk BA, Sikes CR, Farfel GM (2001) Multicenter, double-blind comparison of sertraline and placebo in the treatment of posttraumatic stress disorder. Arch Gen Psychiatry 58:485–492. doi:10.1001/archpsyc.58.5.485
DeMets DL, Lan KK (1994) Interim analysis: the alpha spending function approach. Stats Med 13:1341–1352. doi:10.1002/sim.4780131308
Friedman MJ, Bernardy NC (2016) Considering future pharmacotherapy for PTSD. J Neurosci Lett. doi:10.1016/j.neulet.2016.11.048
Friedman MJ, Davidson JRT (2014) Pharmacotherapy for PTSD. In: Friedman M, Keane T, Resick P (eds) Handbook of PTSD, 2nd edn. Guilford Publications, Inc., New York, pp 482–501
Friedman MJ, Marmar CR, Baker DG, Sikes CR, Farfel GM (2007) Randomized, double-blind comparison of sertraline and placebo for posttraumatic stress disorder in a Department of Veterans Affairs setting. J Clin Psychiatry 68:711–720
Gray MJ, Litz BT, Hsu JL, Lombardo TW (2004) Psychometric properties of the Life Events Checklist. Assessment 11:330–341. doi:10.1177/1073191104269954
Heger M (2013) Trial designs advance to overcome bitter pill of placebo effect. Nat Med 19:1353
Kaminski RM, Livingood MR, Rogawski MA (2004) Allopregnanolone Analogs That Positively Modulate GABAA Receptors Protect against Partial Seizures Induced by 6‐Hz Electrical Stimulation in Mice. Epilepsia 45:864–867
Kay T, Harrington DE, Adams R, Anderson T, Berrol S, Cicerone K et al (1993) Definition of mild traumatic brain injury. J Head Trauma Rehab 8:86–87
King LA, King DW, Vogt DS, Knight JA, Samper R (2006) Deployment Risk and Resilience Inventory: a collection of measures for studying deployment-related experiences of military personnel and veterans. Mil Psychol 18:89–120. doi:10.1207/s15327876mp1802_1
Kroenke K, Spitzer RL, Williams JB (2001) The PHQ-9: validity of a brief depression severity measure. J Gen Int Med 16:606–613. doi:10.1046/j.1525-1497.2001.016009606.x
Kubany ES, Leisen MB, Kaplan AS, Watson SB, Haynes SN, Owens JA et al (2000) Development and preliminary validation of a brief broad-spectrum measure of trauma exposure: the Traumatic Life Events Questionnaire. Psychol Assess 12:210–224. doi:10.1037/1040-3590.12.2.210
Lamba V, Yasuda K, Lamba JK, Assem M, Davila J, Strom S, Schuetz EG. PXR (NR1I2): splice variants in human tissues, including brain, and identification of neurosteroids and nicotine as PXR activators. Toxicol Applied Pharmacol 199:251–65
Lambert JJ, Belelli D, Peden DR, Vardy AW, Peters JA (2003) Neurosteroid modulation of GABA A receptors. Prog. Neurobiology 71:67–80
Laxer K, Blum D, Abou-Khalil BW, Morrell MJ, Lee DA, Data JL et al (2000) Assessment of ganaxolone’s anticonvulsant activity using a randomized, double-blind, presurgical trial design. Ganaxolone Presurgical Study Group. Epilepsia 41:1187–1194. doi:10.1111/j.1528-1157.2000.tb00324.x
Marshall RD, Beebe KL, Oldham M et al (2001) Efficacy and safety of paroxetine treatment for chronic PTSD: a fixed-dose, placebo-controlled study. Am J Psychiatry 158:1982–1988. doi:10.1176/appi.ajp.158.12.1982
Marx CE (2014) Neurosteroids as novel therapeutics and biomarker candidates in TBI, schizophrenia, and PTSD. Inaugural Neurosteroid Congress, Durham
McNair DM, Lorr M, Droppleman LF (1992) Revised manual for the profile of mood states. Educational and Industrial Testing Services, San Diego
Mody I, Pearce RA (2004) Diversity of inhibitory neurotransmission through GABA A receptors. Trends Neurosci 27:569–75
Monaghan EP, Navalta LA, Shum L, Ashbrook DW, Lee DA (1997) Initial human experience with ganaxolone, a neuroactive steroid with antiepileptic activity. Epilepsia. 38:1026–31
Nishith P, Nixon RD, Resick PA (2005) Resolution of trauma-related guilt following treatment of PTSD in female rape victims: A result of cognitive processing therapy targeting comorbid depression? J Affective Disorders 86:259–65
Nohria V, Giller E (2007) Ganaxolone. Neurotherapeutics 4:102–105. doi:10.1016/j.nurt.2006.11.003
Pepe MS, Anderson GL (1992) Two-stage experimental designs: early stopping with a negative result. Appl Stat 41:181–190. doi:10.2307/2347627
Pibiri F, Nelson M, Guidotti A, Costa E, Pinna G (2008) Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: a model relevant for posttraumatic stress disorder. Proc Natl Acad Sci 105:5567–5572. doi:10.1073/pnas.0801853105
Pinna G, Rasmusson AM (2014) Ganaxolone improves behavioral deficits in a mouse model of post-traumatic stress disorder. Front Cell Neurosci 8:1–11. doi:10.3389/fncel.2014.00256
Pinna G, Costa E, Guidotti A (2006) Fluoxetine and norfluoxetine stereospecifically and selectively increase brain neurosteroid content at doses inactive on 5-HT reuptake. Psychopharmacol 24:1–11. doi:10.1007/s00213-005-0213-2
Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Milad MR, Liberzon I (2012) Biological studies of post-traumatic stress disorder. Nature Rev Neurosci 13:769–87
Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, Currier GW, Melvin GA, Greenhill L, Shen S, Mann JJ (2011) The Columbia–Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 168:1266–1277
Rasmusson AM, Abdallah CG (2015) Biomarkers for PTSD treatment and diagnosis. PTSD Res Q 26:1–14
Rasmusson AM, Shalev AY (2014) Integrating the neuroendocrinology, neurochemistry, and neuroimmunology of PTSD to date and the challenges ahead. In Friedman M, Keane T, Resick P (eds) Handbook of PTSD, Second Edition, Guilford Publications, Inc, New York, NY, pp 166–189
Rasmusson AM, Pinna G, Paliwal P, Weisman D, Gottschalk C, Charney DS et al (2006) Decreased cerebrospinal fluid allopregnanolone levels in women with PTSD. Biol Psychiatry 60:704–713. doi:10.1016/j.biopsych.2006.03.026
Rasmusson A, King M, Gregor K, Scioli-Salter E, Pineles S, Valovski I, Hamouda M, Pinna G (2016) Sex differences in the enzyme site at which GABAergic neuroactive steroid synthesis is blocked in PTSD: implications for targeting of PTSD therapeutics. In symposium: sex specificity in posttraumatic stress disorder: from biological mechanisms to treatment response (Fellingham K: Chair; Jovanovich T: Discussant). 32nd Annual Meeting, International Society for Traumatic Stress Studies Dallas
Reddy DS, Castaneda DC, O'Malley BW, Rogawski MA (2004) Anticonvulsant activity of progesterone and neurosteroids in progesterone receptor knockout mice. J Pharmacol Exp Therapeutics. 310:230–9
Resick PA, Monson CM, Gutner CA, Maslej MM (2014) Psychosocial treatments for adults with PTSD. In: Friedman M, Keane T, Resick P (eds) Handbook of PTSD, 2nd edn. Guilford Publications, Inc., New York, pp 419–436
Romeo E, Strohle A, Spalletta G, di Michele F, Hermann B, Holsboer F et al (1998) Effects of antidepressant treatment on neuroactive steroids in major depression. Am J Psychiatry 155:910–913. doi:10.1176/ajp.155.7.910
Schüle C, Nothdurfter C, Rupprecht R (2014) The role of allopregnanolone in depression and anxiety. Prog Neurobiol. doi:10.1016/j.pneurobio.2013.09.003
Semyanov A, Walker MC, Kullmann DM, Silver RA (2004) Tonically active GABA a receptors: modulating gain and maintaining the tone. Trends Neurosci 27:262–269. doi:10.1016/j.tins.2004.03.005
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E et al (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59(Suppl 20):22–33 quiz 34-57
Sinz MW. Pregnane X receptor: prediction and attenuation of human CYP3A4 enzyme induction and drug–drug interactions (2008) Ann Reports Medicinal Chem 43:405–18
Sripada RK, Marx CE, King AP, Rampton JC, Ho SS, Liberzon I (2013) Allopregnanolone elevations following pregnenolone administration are associated with enhanced activation of emotion regulation neurocircuits. Biol Psychiatry. 73:1045–53
Timby E, Balgård M, Nyberg S, Spigset O, Andersson A, Porankiewicz-Asplund J et al (2006) Pharmacokinetic and behavioral effects of allopregnanolone in healthy women. Psychopharmacol 186:414–424. doi:10.1007/s00213-005-0148-7
Tucker P, Zaninelli R, Yehuda R (2001) Paroxetine in the treatment of chronic posttraumatic stress disorder: results of a placebo-controlled, flexible-dosage trial. J Clin Psychiatry 62:860–868
Uzunova V, Sheline Y, Davis J, Rasmusson A, Uzunova D, Costa E et al (1998) Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci. 95:3239–3244.
van Broekhoven F, Bäckström T, van Luijtelaar G, Buitelaar JK, Smits P, Verkes RJ (2007) Effects of allopregnanolone on sedation in men, and in women on oral contraceptives. Psychoneuroendocrinol 32:555–564. doi:10.1016/j.psyneuen.2007.03.009
Zhang LM, Qiu ZK, Zhao N, Chen HX, Liu YQ, Xu JP, Zhang YZ, Yang RF, Li YF (2014) Anxiolytic-like effects of YL-IPA08, a potent ligand for the translocator protein (18 kDa) in animal models of post-traumatic stress disorder. Int J Neuropsychopharmacol 17:1659–1669. doi:10.1017/S1461145714000479
Acknowledgements
INTRuST Consortium lead site principal investigators
Duke University Medical Center: Gerald A. Grant, M.D., and Christine E. Marx, M.D., M.A.;
Spaulding Rehabilitation Hospital: Ross Zafonte, D.O.;
University of California, San Diego: Raul Coimbra, M.D., Ph.D.;
Dartmouth University: Thomas McAllister, M.D.;
Uniformed Services University of the Health Sciences: David Benedek, M.D.;
University of Cincinnati: Lori Shutter, M.D.;
Medical University of South Carolina: Mark S. George, M.D.
Clinical trial performance site principal investigators
VA Medical Center Cincinnati and University of Cincinnati College of Medicine: Thomas Geracioti, M.D.;
Ralph H. Johnson VA Medical Center: Mark Hamner, M.D.;
VA San Diego Healthcare System: James Lohr, M.D.;
Durham VA Medical Center: Christine E. Marx, M.D., M.A.;
VA Boston Healthcare System: Ann M. Rasmusson, M.D.;
Washington DC VA Medical Center: Richard Rosse, M.D.;
Manchester VA Medical Center: Lanier Summerall, M.D.;
White River Junction VA Medical Center: Lanier Summerall, M.D.
INTRuST Consortium clinical trialists and site monitors
Alice L. Mills, M.D., M.P.H., Wendy Ching, Kimberly Aguilar, Karen Stokes, Lisa Kallenberg, M.D., James Payamo, M.D.
Performance site clinical coordinators
Duke University Medical Center Durham VA: Susan O’Loughlin; Research Service, VA Boston Healthcare Service, and Department of Psychiatry, Boston University School of Medicine: Erica R. Scioli-Salter, Ph.D. and Kristin Gregor, Ph.D.; VA Medical Center Cincinnati and University of Cincinnati College of Medicine: Julie Baker-Nolan, MSW and Heather Dodge, MSW; Ralph H. Johnson VA Medical Center: Bridgette Heyward, B.S.; Washington DC VA Medical Center: Erika Roberge, B.S.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
U.S. Army Medical Research and Materiel Command (USAMRMC) Contract # W81XWH-08-2-0159 to: INTRuST Clinical Consortium Coordinating Center, 9500 Gilman Drive, # 0855, La Jolla, CA 92093-0855, Principal Investigator: Murray B. Stein, M.D., M.P.H.
Department of Veterans Affairs Rehabilitation Research and Development.
Career Development Award (1lK2RX000908) to Dr. Jennifer Naylor.
FDA IND# 106,104 & Source of Active and Placebo Medication for Trial
Marinus Pharmaceuticals, Inc.,
21 Business Park Drive,
Branford, Connecticut 06405.
Conflict of interest
Dr. Rasmusson has received compensation as a member of the scientific advisory board for Resilience Therapeutics, Inc., and as a consultant to Cohen Veterans Biosciences. Dr. Marx is an applicant on pending patent applications focused on neurosteroids and derivatives in CNS disorders (no patents issued; no licensing in place; VA 208 waiver issued). As noted in the author affiliations, Drs. Farfel and Tsai were employees of Marinus Pharmaceuticals during the clinical trial; Dr. Farfel is now an employee of Zogenix, Inc.; Dr. Tsai remains an employee of Marinus Pharmaceuticals. Dr. Gericioti was funded as the lead investigator for another DOD-INTRuST consortium clinical trial. He is also a member of the pharmaceutical development company, RxDino, LLC. Dr. Cusin has been a paid consultant to Janssen Pharmaceuticals, Inc. Dr. Stein has been paid in the past 3 years for consulting to Actelion, Janssen, Pfizer and Tonix pharmaceutical companies. He also has been paid for editorial work for UpToDate and the journal Biological Psychiatry, and he is a consultant to Resilience Therapeutics and Oxeia Biopharmaceuticals. Dr. Summerall receives no compensation outside of VA funding and has no conflict of interest. Drs. Hamner, Lohr, Rosse, and Lang have no disclosures.
Additional information
Ann M. Rasmusson and Christine E. Marx share first authorship and are the lead investigators responsible for the design and implementation of the study as submitted for funding and support to the INTRuST Clinical Consortium Coordinating Center.
Where the full trial protocol can be accessed
Marinus Pharmaceuticals, Inc.,
21 Business Park Drive,
Branford, Connecticut 06405
and
INTRuST Clinical Consortium Coordinating Center
9500 Gilman Drive, # 0855
La Jolla, CA 92093–0855
Rights and permissions
About this article
Cite this article
Rasmusson, A.M., Marx, C.E., Jain, S. et al. A randomized controlled trial of ganaxolone in posttraumatic stress disorder. Psychopharmacology 234, 2245–2257 (2017). https://doi.org/10.1007/s00213-017-4649-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-017-4649-y