Abstract
Rationale
Opiate exposure for longer duration develops state of dependence in humans and animals, which is revealed by signs and symptoms of withdrawal precipitated by opioid receptor antagonists. The sudden withdrawal of opioids produces a withdrawal syndrome in opioid-dependent subjects. Insulin and ATP-sensitive potassium (KATP) channel-mediated glucose homeostasis have been shown to modulate morphine withdrawal.
Objective
Present study has been structured to investigate the role of insulin and pharmacological modulator of KATP channel (gliclazide) in experimental morphine withdrawal syndrome, both invivo and invitro.
Methods
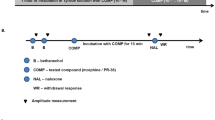
In this study, naloxone-precipitated morphine withdrawal syndrome in mice (invivo) as well as in rat ileum (invitro) were utilized to assess opioid withdrawal phenomenon. Morphine withdrawal syndromes like jumping and rearing frequency, forepaw licking, circling, fore paw tremor, wet dog shake, sneezing, overall morphine withdrawal severity (OMWS), serum glucose, brain malondialdehyde (MDA), glutathione (GSH), nitrite/nitrate, and calcium (Ca+2) were assessed.
Results
Naloxone has significantly increased morphine withdrawal syndrome, both invivo and invitro. Insulin and gliclazide have significantly attenuated, naloxone induced behavioral changes like jumping and rearing frequency, forepaw licking, wet dog shake, sneezing, straightening, circling, OMWS, and various biochemical impairments such as serum glucose, brain MDA, GSH, nitrite/nitrate, and Ca+2 in morphine-dependent animals (invivo). In vitro, insulin and gliclazide have significantly reduced naloxone-induced contraction in morphine-withdrawn rat ileum preparation.
Conclusions
Insulin and gliclazide (KATP channel blocker) have attenuated naloxone-precipitated morphine withdrawal syndrome, both invivo and invitro. Thus, insulin and KATP channel modulation may provide new avenues for research in morphine withdrawal.


Similar content being viewed by others
References
Abdel-Zaher AO, Abdel-Rahman MS, Elwasei FM (2011) Protective effect of Nigella sativa oil against tramadol-induced tolerance and dependence in mice: role of nitric oxide and oxidative stress. Neurotoxicology 32:725–733
Abouazra HA, Sharif SI (2001) Hyperglycaemia: a morphine-like effect produced by naloxone. Clin Exp Pharmacol Physiol 28:300–305
Alp H, Varol S, Celik MM, Altas M, Evliyaoglu O, Tokgoz O, Tanrıverdi MH, Uzar E (2012) Protective effects of beta glucan and gliclazide on brain tissue and sciatic nerve of diabetic rats induced by streptozosin. Exp Diabetes Res. doi:10.1155/2012/230342, PMID: 22291696
Altan VM, Oztürk Y, Yildizoğlu-Ari N, Nebigil C, Lafçi D, Ozçelikay AT (1989) Insulin action on different smooth muscle preparations. Gen Pharmacol 20:529–535
Bi Y, Meng-yin C, Liang H, Sun WP, Chen X, Zhu YH, He XY, Yu QQ, Li M, Weng JP (2009) Effect of early insulin therapy on nuclear factor-kappaB inflammatory pathway in liver of diabetic rat. Zhonghua Nei Ke Za Zhi 48:17–22
Biala G, Betancur C, Mansuy IM, Giros B (2005) The reinforcing effects of chronic D-amphetamine and morphine are impaired in a line of memory-deficient mice overexpressing calcineurin. Eur J Neurosci 21:3089–3096
Biddinger SB, Kahn CR (2006) From mice to men: insights into the insulin resistance syndromes. Annu Rev Physiol 68:123–158
Bongianni F, Carla V, Moroni F, Pellegrini-Giampietro DE (1986) Calcium channel inhibitors suppress the morphine-withdrawal syndrome in rats. Br J Pharmacol 88:561–567
Candell LM, Yun SH, Tran LL, Ehlert FJ (1990) Differential coupling of subtypes of the muscarinic receptor to adenylate cyclase and phosphoinositide hydrolysis in the longitudinal muscle of the rat ileum. Mol Pharmacol 38:689–697
Capasso A, Gallo A (2009) Molecules acting on CB1 receptor and their effects on morphine withdrawal invitro. Open Biochem J 3:78–84
Capasso A, Sorrentino L (1997) Differential influence of D1 and D2 dopamine receptors on acute opiate withdrawal in guinea-pig isolated ileum. Br J Pharmacol 120:1001–1006
Carl A, Burtis, Edward R Ashwood (1996) Mineral and bone metabolism. In: Tietz Fundamentals of Clinical Chemistry. W.B. Saunders, Philadelphia, PA, pp 685–703
Chen LL, Yu F, Zeng TS, Liao YF, Li YM, Ding HC (2011) Effects of gliclazide on endothelial function in patients with newly diagnosed type 2 diabetes. Eur J Pharmacol 659:296–301
Chugh SN, Dhawan R, Kishore K, Sharma A, Chugh K (2001) Glibenclamide vs gliclazide in reducing oxidative stress in patients of noninsulin dependent diabetes mellitus—a double blind randomized study. J Assoc Physicians India 49:803–807
Craft S, Foster TC, Landfield PW, Maier SF, Resnick SM, Yaffe K (2012) Session III. Mechanisms of age-related cognitive change and targets for intervention: inflammatory, oxidative, and metabolic processes. J Gerontol A Biol Sci Med Sci 67:754–759
Cunha TM, Roman-Campos D, Lotufo CM, Duarte HL, Souza GR, Verri WA Jr, Funez MI etal (2010) Morphine peripheral analgesia depends on activation of the PI3Kgamma/AKT/nNOS/NO/KATP signaling pathway. Proc Natl Acad Sci U S A 107:4442–4447
Delaey C, Van de Voorde J (1997) Heterogeneity of the inhibitory influence of sulfonylureas on prostanoid-induced smooth muscle contraction. Eur J Pharmacol 325:41–46
Drdla R, Gassner M, Gingl E, Sandkühler J (2009) Induction of synaptic long-term potentiation after opioid withdrawal. Science 325:207–210
Edwards S, Graham DL, Whisler KN, Self DW (2009) Phosphorylation of GluR1, ERK, and CREB during spontaneous withdrawal from chronic heroin self-administration. Synapse 63:224–235
Enquist J, Ferwerda M, Milan-Lobo L, Whistler JL (2012) Chronic methadone treatment shows a better cost/benefit ratio than chronic morphine in mice. J Pharmacol Exp Ther 340:386–392
Farahmandfar M, Karimian SM, Zarrindast MR, Kadivar M, Afrouzi H, Naghdi N (2011) Morphine sensitization increases the extracellular level of glutamate in CA1 of rat hippocampus via μ-opioid receptor. Neurosci Lett 494:130–134
Ferenczi S, Núñez C, Pintér-Kübler B, Földes A, Martín F, Márkus VL, Milanés MV, Kovács KJ (2010) Changes in metabolic-related variables during chronic morphine treatment. Neurochem Int 57:323–330
Griffin MT, Ehlert FJ (1992) Specific inhibition of isoproterenol-stimulated cyclic AMP accumulation by M2 muscarinic receptors in rat intestinal smooth muscle. J Pharmacol Exp Ther 263:221–225
Guerrero-Munóz F, Guerrero ML, Way EL (1979) Effect of morphine on calcium uptake by lysed synaptosomes. J Pharmacol Exp Ther 211:370–374
Haba M, Hatakeyama N, Kinoshita H, Teramae H, Azma T, Hatano Y, Matsuda N (2010) The modulation of vascular ATP-sensitive K+ channel function via the phosphatidylinositol 3-kinase-Akt pathway activated by phenylephrine. J Pharmacol Exp Ther 334:673–678
Højsted J, Kurita GP, Kendall S, Lundorff L, de Mattos Pimenta CA, Sjøgren P (2012) Non-analgesic effects of opioids: the cognitive effects of opioids in chronic pain of malignant and non-malignant origin. An update. Curr Pharm Des 18:6116–6122
Ipp E, Schusdziarra V, Harris V, Unger RH (1980) Morphine-induced hyperglycemia: role of insulin and glucagon. Endocrinology 107:461–463
Jafari MR, Zarrindast MR, Djahanguiri B (2004) Effects of different doses of glucose and insulin on morphine state-dependent memory of passive avoidance in mice. Psychopharmacology (Berl) 175:457–462
Jarrard RE, Wang Y, Salyer AE, Pratt EP, Soderling IM, Guerra ML, Lange AM, Broderick HJ, Hockerman GH (2013) Potentiation of sulfonylurea action by an EPAC-selective cAMP analog in INS-1 cells: comparison of tolbutamide and gliclazide and a potential role for EPAC activation of a 2-APB-sensitive Ca2+ influx. Mol Pharmacol 83:191–205
Johansen O, Tønnesen T, Jensen T, Burhol PG, Jorde R, Reikerås O (1993) Morphine and morphine/naloxone modification of glucose, glucagon and insulin levels in fasted and fed rats. Scand J Clin Lab Invest 53:805–809
Kaplan M, Aviram M, Hayek T (2012) Oxidative stress and macrophage foam cell formation during diabetes mellitus-induced atherogenesis: role of insulin therapy. Pharmacol Ther 136:175–185
Kitamura T, Sato K, Kawamura G, Yamada Y (2012) The involvement of adenosine triphosphate-sensitive potassium channels in the different effects of sevoflurane and propofol on glucose metabolism in fed rats. Anesth Analg 114:110–116
Koch T, Seifert A, Wu DF, Rankovic M, Kraus J, Börner C, Brandenburg LO, Schröder H, Höllt V (2009) mu-opioid receptor-stimulated synthesis of reactive oxygen species is mediated via phospholipase D2. J Neurochem 110:1288–1296
Kumar N, Dey CS (2002) Gliclazide increases insulin receptor tyrosine phosphorylation but not p38 phosphorylation in insulin-resistant skeletal muscle cells. J Exp Biol 205:3739–3746
Lee FA, Baiamonte BA, Spano D, Lahoste GJ, Soignier RD, Harrison LM (2011) Mice lacking rhes show altered morphine analgesia, tolerance, and dependence. Neurosci Lett 489:182–186
Li Y, Eitan S, Wu J, Evans CJ, Kieffer B, Sun X, Polakiewicz RD (2003) Morphine induces desensitization of insulin receptor signaling. Mol Cell Biol 23:6255–6266
Li H, Liu B, Huang J, Chen H, Guo X, Yuan Z (2013) Insulin inhibits lipopolysaccharide-induced nitric oxide synthase expression in rat primary astrocytes. Brain Res 1506:1–11
Liang DY, Li X, Clark JD (2013) Epigenetic regulation of opioid-induced hyperalgesia, dependence, and tolerance in mice. J Pain 14:36–47
Liu D, Pitta M, Lee JH, Ray B, Lahiri DK, Furukawa K, Mughal M, Jiang H etal (2010) The KATP channel activator diazoxide ameliorates amyloid-β and tau pathologies and improves memory in the 3 × TgAD mouse model of Alzheimer’s disease. J Alzheimers Dis 22:443–457
Liu L, Coller JK, Watkins LR, Somogyi AA, Hutchinson MR (2011) Naloxone-precipitated morphine withdrawal behavior and brain IL-1β expression: comparison of different mouse strains. Brain Behav Immun 25:1223–1232
Mazzucchelli C, Vantaggiato C, Ciamei A, Fasano S, Pakhotin P, Krezel W, Welzl H, Wolfer DP etal (2002) Knockout of ERK1 MAP kinase enhances synaptic plasticity in the striatum and facilitates striatal-mediated learning and memory. Neuron 34:807–820
Mesripour A, Iyer A, Brown L (2012) Mineralocorticoid receptors mediate cardiac remodelling in morphine-dependent rats. Basic Clin Pharmacol Toxicol 111:75–80
Moon MJ, Kim HY, Park S, Kim DK, Cho EB, Hwang JI, Seong JY (2011) Insulin contributes to fine-tuning of the pancreatic beta-cell response to glucagon-like peptide-1. Mol Cells 32:389–395
Muller DL, Unterwald EM (2004) In vivo regulation of extracellular signal-regulated protein kinase (ERK) and protein kinase B (Akt) phosphorylation by acute and chronic morphine. J Pharmacol Exp Ther 310:774–782
Mundey MK, Mason R, Wilson VG (1998) Selective potentiation by ouabain of naloxone-induced withdrawal contractions of isolated guinea-pig ileum following acute exposure to morphine. Br J Pharmacol 124:911–916
Ohnota H, Koizumi T, Tsutsumi N, Kobayashi M, Inoue S, Sato F (1994) Novel rapid- and short-acting hypoglycemic agent, a calcium(2 s)-2-benzyl-3-(cis-hexahydro-2-isoindolinylcarbonyl) propionate (KAD-1229) that acts on the sulfonylurea receptor: comparison of effects between KAD-1229 and gliclazide. J Pharmacol Exp Ther 269:489–495
Onozato ML, Tojo A, Goto A, Fujita T (2004) Radical scavenging effect of gliclazide in diabetic rats fed with a high cholesterol diet. Kidney Int 65:951–960
Ortiz MI, Fernández-Martínez E, Ponce-Monter H, Pérez-Hernández N, Macías A, Rangel-Flores E, Castañeda-Hérnandez G (2007) The peripheral antinociceptive effect of nalbuphine is associated with activation of ATP-sensitive K+ channels. Proc West Pharmacol Soc 50:72–74
Park SH, Sim YB, Kang YJ, Kim SM, Lee JK, Jung JS, Suh HW (2010) Characterization of blood glucose level regulation in mouse opioid withdrawal models. Neurosci Lett 476:119–122
Pateliya BB, Singh N, Jaggi AS (2008) Possible role of opioids and KATP channels in neuroprotective effect of postconditioning in mice. Biol Pharm Bull 31:1755–1760
Pfister SL, Pratt PE, Kurian J, Campbell WB (2004) Glibenclamide inhibits thromboxane-mediated vasoconstriction by thromboxane receptor blockade. Vascul Pharmacol 40:285–292
Pinelli A, Cighetti G, Trivulzio S (2008) Plasma malondialdehyde levels and opiate withdrawal signs observed in rats treated with morphine plus naloxone: effects of alpha-lipoic acid administration. Fundam Clin Pharmacol 22(4):439–445
Pinelli A, Cighetti G, Trivulzio S (2009) Effects of alpha-lipoic acid administration on plasma glucose levels, total malondialdehyde values and withdrawal signs in rats treated with morphine or morphine plus naloxone. Arzneimittelforschung 59:72–78
Qi R, Ozaki Y, Satoh K, Kurota K, Asazuma N, Yatomi Y, Kume S (1995) Sulphonylurea agents inhibit platelet aggregation and [Ca2+]i elevation induced by arachidonic acid. Biochem Pharmacol 49:1735–1739
Radosevich PM, Williams PE, Lacy DB, McRae JR, Steiner KE, Cherrington AD, Lacy WW, Abumrad NN (1984) Effects of morphine on glucose homeostasis in the conscious dog. J Clin Invest 74:1473–1480
Rehni AK, Singh N (2012) Ammonium pyrrolidine dithiocarbamate and RS 102895 attenuate opioid withdrawal invivo and invitro. Psychopharmacology (Berl) 220:427–438
Rehni AK, Singh N, Rachamalla M, Tikoo K (2012) Modulation of histone deacetylase attenuates naloxone-precipitated opioid withdrawal syndrome. Naunyn Schmiedebergs Arch Pharmacol 385:605–619
Ross DH, Kibler BC, Cardenas HL (1977) Modification of glycoprotein residues as Ca2+ receptor sites after chronic ethanol exposure. Drug Alcohol Depend 2:305–315
Saltiel AR, Kahn CR (2001) Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414:799–806
Samandari R, Chizari A, Hassanpour R, Mousavi Z, Haghparast A (2013) Streptozotocin-induced diabetes affects the development and maintenance of morphine reward in rats. Neurosci Lett 543:90–94
Satyanarayana S, Kilari EK (2006) Influence of nicorandil on the pharmacodynamics and pharmacokinetics of gliclazide in rats and rabbits. Mol Cell Biochem 291:101–105
Sharma B, Singh N (2010) Pitavastatin and 4′-hydroxy-3′-methoxyacetophenone (HMAP) reduce cognitive dysfunction in vascular dementia during experimental diabetes. Curr Neurovasc Res 7:180–191
Sharma B, Singh N (2011a) Attenuation of vascular dementia by sodium butyrate in streptozotocin diabetic rats. Psychopharmacology (Berl) 215:677–687
Sharma B, Singh N (2011b) Behavioral and biochemical investigations to explore pharmacological potential of PPAR-gamma agonists in vascular dementia of diabetic rats. Pharmacol Biochem Behav 100:320–329
Sharma B, Singh N (2012a) Salutary effect of NFκB inhibitor and folacin in hyperhomocysteinemia–hyperlipidemia induced vascular dementia. Prog Neuropsychopharmacol Biol Psychiatry 38:207–215
Sharma B, Singh N (2012b) Experimental hypertension induced vascular dementia: pharmacological, biochemical and behavioral recuperation by angiotensin receptor blocker and acetylcholinesterase inhibitor. Pharmacol Biochem Behav 102:101–108
Sharma B, Singh N (2012c) Defensive effect of natrium diethyldithiocarbamate trihydrate (NDDCT) and lisinopril in DOCA-salt hypertension-induced vascular dementia in rats. Psychopharmacology (Berl) 223:307–317
Sharma B, Singh N (2013) Pharmacological inhibition of inducible nitric oxide synthase (iNOS) and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, convalesce behavior and biochemistry of hypertension induced vascular dementia in rats. Pharmacol Biochem Behav 103:821–830
Sharma B, Singh N, Singh M (2008a) Modulation of celecoxib- and streptozotocin-induced experimental dementia of Alzheimer’s disease by pitavastatin and donepezil. J Psychopharmacol 22:162–171
Sharma B, Singh N, Singh M, Jaggi AS (2008b) Exploitation of HIV protease inhibitor Indinavir as a memory restorative agent in experimental dementia. Pharmacol Biochem Behav 89:535–545
Shen KZ, Johnson SW (2010) Ca2+ influx through NMDA-gated channels activates ATP-sensitive K+ currents through a nitric oxide-cGMP pathway in subthalamic neurons. J Neurosci 30:1882–1893
Simpkins JW, Katovich MJ, Millard WJ (1990) Glucose modulation of skin temperature responses during morphine withdrawal in the rat. Psychopharmacology (Berl) 102:213–220
Sliwinska A, Rogalska A, Szwed M, Kasznicki J, Jozwiak Z, Drzewoski J (2012) Gliclazide may have an antiapoptotic effect related to its antioxidant properties in human normal and cancer cells. Mol Biol Rep 39:5253–5267
Surwit RS, McCubbin JA, Kuhn CM, Cochrane C, Feinglos MN (1989) Differential glycemic effects of morphine in diabetic and normal mice. Metabolism 38:282–285
Thomson MJ, Williams MG, Frost SC (1997) Development of insulin resistance in 3 T3-L1 adipocytes. J Biol Chem 272:7759–7764
Torres HN, Flawià MM, Hernaez L, Cuatrecasas P (1978) Effects of insulin on the adenylyl cyclase activity of isolated fat cell membranes. J Membr Biol 43:1–18
Valeri P, Morrone LA, Romanelli L, Amico MC (1995) Acute withdrawal after bremazocine and the interaction between mu- and kappa-opioid receptors in isolated gut tissues. Br J Pharmacol 114:1206–1210
Waxman EA, Lynch DR (2005) N-methyl-d-aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist 11:37–49
Way EL, Loh HH, Shen FH (1969) Simultaneous quantitative assessment of morphine tolerance and physical dependence. J Pharmacol Exp Ther 167:1–8
Wen T, Peng B, Pintar JE (2009) The MOR-1 opioid receptor regulates glucose homeostasis by modulating insulin secretion. Mol Endocrinol 23:671–678
Williams CA, Shih MF, Taberner PV (2000) Effect of acute and sub-chronic administration of the imidazoline compound S 22068 on invivo glucose and insulin responses in normal lean CBA/Ca mice. Gen Pharmacol 34:183–191
Yamamoto H, Harris RA, Loh HH, Way EL (1978) Effects of acute and chronic morphine treatments on calcium localization and binding in brain. J Pharmacol Exp Ther 205:255–264
Yang Y, Cheng Y, Lian QQ, Yang L, Qi W, Wu DR, Zheng X, Liu YJ etal (2013) Contribution of CFTR to alveolar fluid clearance by lipoxin A 4 via PI3K/Akt pathway in LPS-induced acute lung injury. Mediators Inflamm 2013:862628
Zachariou V, Liu R, LaPlant Q, Xiao G, Renthal W, Chan GC, Storm DR, Aghajanian G, Nestler EJ (2008) Distinct roles of adenylyl cyclases 1 and 8 in opiate dependence: behavioral, electrophysiological, and molecular studies. Biol Psychiatry 63:1013–1021
Zhou J, Li Y, Yan G, Bu Q, Lv L, Yang Y, Zhao J, Shao X, Deng Y, Zhu R, Zhao Y, Cen X (2011) Protective role of taurine against morphine-induced neurotoxicity in C6 cells via inhibition of oxidative stress. Neurotox Res 20:334–342
Acknowledgements
The authors are thankful to the Space Age Research and Technical Foundation Charitable Trust (SPRFCT), Bharat Institute of Technology, Meerut, India, for providing all the necessary facilities and funding to conduct this research work. We are also thankful to Dr. Nirmal Singh, Associate Professor, Pharmacology Division, Department of Pharmaceutical Sciences and Drug Research, Faculty of Medicine, Punjabi University, Patiala, Punjab, India, for his valuable suggestions.
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 662 kb)
Rights and permissions
About this article
Cite this article
Singh, P., Sharma, B., Gupta, S. et al. In vivo and in vitro attenuation of naloxone-precipitated experimental opioid withdrawal syndrome by insulin and selective KATP channel modulator. Psychopharmacology 232, 465–475 (2015). https://doi.org/10.1007/s00213-014-3680-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-014-3680-5