Abstract
Rationale
Previous open-label studies have suggested that quetiapine could be a valuable alternative for treating fibromyalgia.
Objective
This study aims to compare the efficacy and tolerability of extended-release quetiapine with amitriptyline for treating fibromyalgia.
Methods
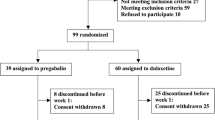
This study was a randomized, open-label, flexible-dose, non-inferiority trial. Patients with fibromyalgia were randomized to receive quetiapine extended-release (XR) (N = 45) (50 to 300 mg daily) or amitriptyline (N = 45) (10 to 75 mg daily) for 16 weeks. The primary endpoint was the change from baseline to endpoint in the Fibromyalgia Impact Questionnaire (FIQ) total score; the non-inferiority threshold was established at 8 points. The secondary outcomes included sleep quality, anxiety, depression, and quality of life.
Results
Twenty-two (49 %) patients in the quetiapine group and 34 (76 %) patients in the amitriptyline group completed the study. We found a reduction of 9.8 points in the total FIQ score at the endpoint for the quetiapine-treated patients compared to 13.9 points for the amitriptyline-treated patients, for a difference of 4.14 points (80 % confidence interval (CI) −0.70 to 8.98). No significant differences were found between the quetiapine XR and amitriptyline groups for any of the secondary outcomes. The proportion of patients discontinuing treatment due to adverse events was higher in the quetiapine group (n = 14, 31.1 %) than the amitriptyline group (n = 3, 6.6 %).
Conclusions
Our results appear to indicate that quetiapine XR does not provide similar efficacy to amitriptyline for treating patients with fibromyalgia. Quetiapine XR had a worse tolerability than amitriptyline in this population, possibly due to a relatively high starting dose.



Similar content being viewed by others
References
Alonso J, Prieto L, Antó JM (1995) The Spanish version of the SF-36 Health Survey (the SF-36 health questionnaire): an instrument for measuring clinical results. Med Clin (Barc) 104:771–776
Badia X, Muriel C, Gracia A et al (2003) Validación española del cuestionario Brief Pain Inventory en pacientes con dolor de causa neoplásica. Med Clin (Barc) 120:52–59
Bandelow B, Chouinard G, Bobes J et al (2010) Extended-quetiapine fumarate (quetiapine XR): a one-daily monotherapy effective in generalized anxiety disorder. Data from a randomized, double-blind, placebo- and active-controlled study. Int J Neuropsychopharmacol 13:305–320
Barkhuizen A (2002) Rational and targeted pharmacologic treatment of fibromyalgia. Rheum Dis Clin North Am 28:261–290
Boyle J, Eriksson ME, Gribble L et al (2012) Randomized, placebo-controlled comparison of amitriptyline, duloxetine, and pregabalin in patients with chronic diabetic peripheral neuropathic pain: impact on pain, polysomnographic sleep, daytime functioning, and quality of life. Diabetes Care 35:2451–2458
Calandre EP, Rico-Villademoros F (2012) The role of antipsychotics in the management of fibromyalgia. CNS Drugs 6:135–153
Calandre EP, Morillas-Arques P, Molina-Barea R et al (2011) Trazodone plus pregabalin combination in the treatment of fibromyalgia: a two-phase, 24-week, open-label uncontrolled study. BMC Musculoskeletal Disord 12:95
Coe HV, Hong IS (2012) Safety of low doses of quetiapine when used for insomnia. Ann Pharmacother 46:718–22
Cohrs S, Rodenbeck A, Guan Z et al (2004) Sleep-promoting properties of quetiapine in healthy subjects. Psychopharmacol (Berl) 174:421–429
Esteve-Vives J, Rivera Redondo J, Salvat Salvat MI et al (2007) Propuesta de una versión de consenso del Fibromyalgia Impact Questionnaire (FIQ) para la población española. Reumatol Clin 3:21–24
Fisher DS, Handley SA, Flanagan RJ et al (2012) Plasma concentrations of quetiapine, N-desalkylquetiapine, o-desalkylquetiapine, 7-hydroxyquetiapine, and quetiapine sulfoxide in relation to quetiapine dose, formulation and other factors. Ther Drug Monit 34:415–421
Gao K, Kemp DE, Fein E et al (2011) Number needed to treat to harm for discontinuation due to adverse events in the treatment of bipolar depression, major depressive disorder, and generalized anxiety disorder with atypical antipsychotics. J Clin Psychiatry 72:1063–1071
Guy W (1976) ECDEU assessment manual for psychopharmacology—revised (DHEW Publ No ADM 76-338). U.S. Department of Health, Rockville
Häuser W, Petzke F, Sommer C (2010) Comparative efficacy and harms of duloxetine, milnacipran and pregabalin in fibromyalgia syndrome. J Pain 11:505–521
Häuser W, Wolfe F, Tölle T et al (2012) The role of antidepressants in the management of fibromyalgia syndrome: a systematic review and meta-analysis. CNS Drugs 26:297–307
Hidalgo J, Rico-Villademoros F, Calandre EP (2007) An open-label study of quetiapine in the treatment of fibromyalgia. Prog Neuropsychopharmacol Biol Psychiatry 31:71–77
Katzman MA, Brawman-Mintzer O, Reyes EB et al (2011) Extended-release quetiapine fumarate (quetiapine XR) monotherapy as maintenance treatment for generalized anxiety disorder: a long-term, randomized, placebo-controlled trial. Int Clin Psychopharmacol 26:11–24
Le Marshall KF, Littlejohn GO (2011) Management strategies for fibromyalgia. Open Access Rheumatol Res Rev 3:47–51
McIntyre RS, Soczynska JK, Woldeyoannes HO et al (2007) A preclinical and clinical rationale for quetiapine in mood syndromes. Expert Opin Pharmacother 8:1211–1219
McIntyre A, Paisley D, Kuoassi E, Gendron A (2013) Quetiapine fumarate extended release or the treatment of major depression with comorbid fibromyalgia syndrome a double-blind, randomized, placebo controlled study. Arthritis Rheum. doi:10.1002/art38228
Moore N, McMillan P, Birur B, et al. (2011) Fibromyalgia: efficacy of quetiapine compared with placebo [Abstract no. NR08–48:333]. In 164th annual meeting of the American Psychiatric Association. Honolulu, Hawaii
Potvin S, Morin M, Cloutier C et al (2012) Add-on treatment of quetiapine for fibromyalgia. A pilot, randomized, double-blind, placebo-controlled 12-week trial. J Clin Psychopharmacol 2:684–687
Royuela A, Macías JA (1997) Propiedades clinimétricas de la versión castellana del cuestionario de Pittsburgh. Vigilia-Sueño 9:81–94
Shemo M, Shemo JPD, Anderson M (2003) Beneficial effects of quetiapine treatment in patients with fibromyalgia [Abstract]. J Neuropsychiatry Clin Neurosci 15:282
Spielberger CD, Gorsuch RL, Lushene RE (2002) STAI Cuestionario de Ansiedad Estado-Rasgo. Manual (6ª edición). Publicaciones de Psicología Aplicada. Serie menor número 124. TEA Ediciones S.A, Madrid
Vazquez C, Sanz J (1997) Fiabilidad y valores normales de la versión española del inventario para la depresión de Beck de 1978. Clin Salud 8:403–422
Wang Z, Kemp DE, Chan PK et al (2011) Comparisons of the tolerability and sensitivity of quetiapine-XR in the acute treatment of schizophrenia, bipolar mania, bipolar depression, major depressive disorder, and generalized anxiety disorder. Int J Neuropsychopharmacol 14:131–142
Wolfe F, Smythe HA, Yunus MB et al (1990) The American College of Rheumatology 1990 criteria for the classification of fibromyalgia: report of the multicenter criteria committee. Arthritis Rheum 33:160–172
Wu CH, Farley JF, Gaynes BN (2012) The association between antidepressant dosage titration and medication adherence among patients with depression. Depress Anxiety 29:506–514
Acknowledgments
Partial funding for this study was provided by AstraZeneca, as an investigator-sponsored study. The company had no involvement in writing the manuscript or interpreting the results.
Conflict of interest
Dr. Rico-Villademoros has served as a freelance consultant for AstraZeneca Farmacéutica Spain. The remaining authors do not declare any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Calandre, E.P., Rico-Villademoros, F., Galán, J. et al. Quetiapine extended-release (Seroquel-XR) versus amitriptyline monotherapy for treating patients with fibromyalgia: a 16-week, randomized, flexible-dose, open-label trial. Psychopharmacology 231, 2525–2531 (2014). https://doi.org/10.1007/s00213-013-3422-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-3422-0