Abstract
Rationale
Several recent studies have focused on glutamate modulating agents for symptoms relief in schizophrenia, especially negative symptoms which are resistant to conventional therapies.
Objectives
We aimed to assess the efficacy and tolerability of riluzole, an anti-glutamate agent with neuroprotective properties, as an adjunct to risperidone in improving negative symptoms of schizophrenia.
Methods
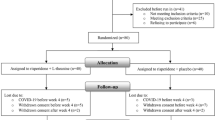
In this randomized double-blind placebo-controlled parallel-group study, 50 patients with chronic schizophrenia and a score of ≥20 on the negative subscale of positive and negative syndrome scale (PANSS) were enrolled in the active phase of their illness. Participants were equally randomized to receive riluzole (100 mg/day) or placebo in addition to risperidone (up to 6 mg/day) for 8 weeks. Participants were rated by PANSS every 2 weeks. The primary outcome of this study was the difference in the decrease of PANSS negative subscale score from baseline to the study endpoint between the two groups.
Results
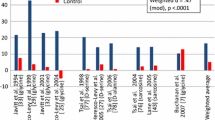
By the study endpoint, riluzole-treated patients showed significantly greater improvement in the negative symptoms (P < 0.001) as well as the PANSS total and general psychopathology subscale scores (P = 0.001 and P < 0.001; respectively) compared to the placebo group. Treatment group was the only significant predictor of changes in negative symptom in this trial (β = −0.56, P < 0.001). No significant difference was observed between two groups in the frequency of side effects.
Conclusion
These preliminary findings suggest that riluzole may be a safe and effective medication for the treatment of negative symptoms in patients with chronic schizophrenia. Further research and replication of study findings is warranted.
Clinical trial registry name and registration number
Iranian registry of clinical trials www.irct.ir, IRCT201107281556N26





Similar content being viewed by others
References
Akhondzadeh S, Ghayyoumi R, Rezaei F, Salehi B, Modabbernia AH, Maroufi A, Esfandiari GR, Naderi M, Ghebleh F, Tabrizi M, Rezazadeh SA (2011) Sildenafil adjunctive therapy to risperidone in the treatment of the negative symptoms of schizophrenia: a double-blind randomized placebo-controlled trial. Psychopharmacology (Berl) 213:809–815
Anand A, Charney DS, Oren DA, Berman RM, Hu XS, Cappiello A, Krystal JH (2000) Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: support for hyperglutamatergic effects of N-methyl-D-aspartate receptor antagonists. Arch Gen Psychiatry 57:270–276
Arundine M, Tymianski M (2003) Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell calcium 34:325–337
Chouinard G, Margolese HC (2005) Manual for the extrapyramidal symptom rating scale (ESRS). Schizophr Res 76:247–265
Coric V, Taskiran S, Pittenger C, Wasylink S, Mathalon DH, Valentine G, Saksa J, Wu YT, Gueorguieva R, Sanacora G, Malison RT, Krystal JH (2005) Riluzole augmentation in treatment-resistant obsessive-compulsive disorder: an open-label trial. Biol Psychiatry 58:424–428
Coyle JT (2006) Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol 26:365–384
Crow TJ (1981) Positive and negative schizophrenia symptoms and the role of dopamine. Br J Psychiatry 139:251–254
Danysz W, Parsons CG (2002) Neuroprotective potential of ionotropic glutamate receptor antagonists. Neurotox Res 4:119–126
Doble A (1996) The pharmacology and mechanism of action of riluzole. Neurology 47:S233–S241
Du J, Suzuki K, Wei Y, Wang Y, Blumenthal R, Chen Z, Falke C, Zarate CA Jr, Manji HK (2007) The anticonvulsants lamotrigine, riluzole, and valproate differentially regulate AMPA receptor membrane localization: relationship to clinical effects in mood disorders. Neuropsychopharmacology 32:793–802
Durany N, Thome J (2004) Neurotrophic factors and the pathophysiology of schizophrenic psychoses. Eur Psychiatry 19:326–337
Fenton WS, McGlashan TH (1991) Natural history of schizophrenia subtypes. II. Positive and negative symptoms and long-term course. Arch Gen Psychiatry 48:978–986
Frizzo ME, Dall’Onder LP, Dalcin KB, Souza DO (2004) Riluzole enhances glutamate uptake in rat astrocyte cultures. Cell Mol Neurobiol 24:123–128
Ghaleiha A, Mohammadi E, Mohammadi MR, Farokhnia M, Modabbernia A, Yekehtaz H, Ashrafi M, Hassanzadeh E, Akhondzadeh S (2013) Riluzole as an adjunctive therapy to risperidone for the treatment of irritability in children with autistic disorder: a double-blind, placebo-controlled. Randomized Trial, Paediatr Drugs. doi:10.1111/bdi.12108
Gilgun-Sherki Y, Panet H, Melamed E, Offen D (2003) Riluzole suppresses experimental autoimmune encephalomyelitis: implications for the treatment of multiple sclerosis. Brain Res 989:196–204
Grant P, Lougee L, Hirschtritt M, Swedo SE (2007) An open-label trial of riluzole, a glutamate antagonist, in children with treatment-resistant obsessive-compulsive disorder. J Child Adolesc Psychopharmacol 17:761–767
Grant P, Song JY, Swedo SE (2010) Review of the use of the glutamate antagonist riluzole in psychiatric disorders and a description of recent use in childhood obsessive-compulsive disorder. J Child Adolesc Psychopharmacol 20:309–315
Hamilton M (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62
Hanson E, Healey K, Wolf D, Kohler C (2010) Assessment of pharmacotherapy for negative symptoms of schizophrenia. Curr Psychiatry Rep 12:563–571
Javitt DC (2012) Twenty-five years of glutamate in schizophrenia: are we there yet? Schizophr Bull 38:911–913
Jedema HP, Moghaddam B (1994) Glutamatergic control of dopamine release during stress in the rat prefrontal cortex. J Neurochem 63:785–788
Jedema HP, Moghddam B (1996) Characterization of excitatory amino acid modulation of dopamine release in the prefrontal cortex of conscious rats. J Neurochem 66:1448–1453
Jentsch JD, Redmond DE, Jr., Elsworth JD, Taylor JR, Youngren KD, Roth RH (1997) Enduring cognitive deficits and cortical dopamine dysfunction in monkeys after long-term administration of phencyclidine. Science 277: 953–955.
Jentsch JD, Roth RH (1999) The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology 20:201–225
Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276
Khodaie-Ardakani MR, Seddighi S, Modabbernia A, Rezaei F, Salehi B, Ashrafi M, Shams-Alizadeh N, Mohammad-Karimi M, Esfandiari GR, Hajiaghaee R, Akhondzadeh S (2013) Granisetron as an add-on to risperidone for treatment of negative symptoms in patients with stable schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res 47:472–478
Krystal JH, D’Souza DC, Mathalon D, Perry E, Belger A, Hoffman R (2003) NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology 169:215–233
Lacomblez L, Bensimon G, Leigh PN, Debove C, Bejuit R, Truffinet P, Meininger V (2002) Long-term safety of riluzole in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 3:23–29
Laruelle M, Frankle WG, Narendran R, Kegeles LS, Abi-Dargham A (2005) Mechanism of action of antipsychotic drugs: from dopamine D(2) receptor antagonism to glutamate NMDA facilitation. Clin Ther 27 Suppl A: S16–S24
Lourenco Da Silva A, Hoffmann A, Dietrich MO, Dall’Igna OP, Souza DO, Lara DR (2003) Effect of riluzole on MK-801 and amphetamine-induced hyperlocomotion. Neuropsychobiology 48:27–30
Martin D, Thompson MA, Nadler JV (1993) The neuroprotective agent riluzole inhibits release of glutamate and aspartate from slices of hippocampal area CA1. Eur J Pharmacol 250:473–476
Mathew SJ, Amiel JM, Coplan JD, Fitterling HA, Sackeim HA, Gorman JM (2005) Open-label trial of riluzole in generalized anxiety disorder. Am J Psychiatry 162:2379–2381
Miller RG, Mitchell JD, Moore DH (2012) Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane database of systematic reviews (Online) 3: CD001447.
Mizuta I, Ohta M, Ohta K, Nishimura M, Mizuta E, Kuno S (2001) Riluzole stimulates nerve growth factor, brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor synthesis in cultured mouse astrocytes. Neurosci Lett 310:117–120
Modabbernia A, Rezaei F, Salehi B, Jafarinia M, Ashrafi M, Tabrizi M, Hosseini SM, Tajdini M, Ghaleiha A, Akhondzadeh S (2013) Intranasal oxytocin as an adjunct to risperidone in patients with schizophrenia: an 8-week, randomized, double-blind, placebo-controlled study. CNS Drugs 27:57–65
Moghaddam B (2004) Targeting metabotropic glutamate receptors for treatment of the cognitive symptoms of schizophrenia. Psychopharmacology 174:39–44
Moghaddam B, Adams B, Verma A, Daly D (1997) Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. Journal Neurosci 17:2921–2927
Moghaddam B, Adams BW (1998) Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science 281:1349–1352.
Muller N, Schwarz MJ (2010) Immune System and Schizophrenia. Curr Immunol Rev 6:213–220
Murphy BP, Chung YC, Park TW, McGorry PD (2006) Pharmacological treatment of primary negative symptoms in schizophrenia: a systematic review. Schizophr Res 88:5–25
Noroozian M, Ghasemi S, Hosseini SM, Modabbernia A, Khodaie-Ardakani MR, Mirshafiee O, Farokhnia M, Tajdini M, Rezaei F, Salehi B, Ashrafi M, Yekehtaz H, Tabrizi M, Akhondzadeh S (2013) A placebo-controlled study of tropisetron added to risperidone for the treatment of negative symptoms in chronic and stable schizophrenia. Psychopharmacology (Berl) 228(4):595–602
Paz RD, Tardito S, Atzori M, Tseng KY (2008) Glutamatergic dysfunction in schizophrenia: from basic neuroscience to clinical psychopharmacology. Eur neuropsychopharmacol 18:773–786
Rezaei F, Mohammad-Karimi M, Seddighi S, Modabbernia A, Ashrafi M, Salehi B, Hammidi S, Motasami H, Hajiaghaee R, Tabrizi M, Akhondzadeh S (2013) Memantine add-on to risperidone for treatment of negative symptoms in patients with stable schizophrenia: randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol 33:336–342
Sanacora G, Kendell SF, Levin Y, Simen AA, Fenton LR, Coric V, Krystal JH (2007) Preliminary evidence of riluzole efficacy in antidepressant-treated patients with residual depressive symptoms. Biol Psychiatry 61:822–825
Stone JM, Morrison PD, Pilowsky LS (2007) Glutamate and dopamine dysregulation in schizophrenia—a synthesis and selective review. J Psychopharmacol 21:440–452
Wang SJ, Wang KY, Wang WC (2004) Mechanisms underlying the riluzole inhibition of glutamate release from rat cerebral cortex nerve terminals (synaptosomes). Neuroscience 125:191–201
Zarate CA Jr, Payne JL, Quiroz J, Sporn J, Denicoff KK, Luckenbaugh D, Charney DS, Manji HK (2004) An open-label trial of riluzole in patients with treatment-resistant major depression. Am J Psychiatry 161:171–174
Zarate CA Jr, Quiroz JA, Singh JB, Denicoff KD, De Jesus G, Luckenbaugh DA, Charney DS, Manji HK (2005) An open-label trial of the glutamate-modulating agent riluzole in combination with lithium for the treatment of bipolar depression. Biol Psychiatry 57:430–432
Zarate CA, Manji HK (2008) Riluzole in psychiatry: a systematic review of the literature. Expert Opin Drug Metab Toxicol 4:1223–1234
Acknowledgements
This study was supported by a grant from Tehran University of Medical Sciences to Prof. Shahin Akhondzadeh (grant no. 14037). This study was Dr. Maryam Sabzabadi’s postgraduate thesis toward the Iranian Board of Psychiatry.
Conflict of interest
No conflict of interest exists for any of the authors associated with the manuscript and there was no source of extra-institutional commercial funding. The funding organization had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript and the decision to submit the paper for publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farokhnia, M., Sabzabadi, M., Pourmahmoud, H. et al. A double-blind, placebo controlled, randomized trial of riluzole as an adjunct to risperidone for treatment of negative symptoms in patients with chronic schizophrenia. Psychopharmacology 231, 533–542 (2014). https://doi.org/10.1007/s00213-013-3261-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-3261-z