Abstract
Rationale
Thyroid hormones and their interactions with catecholamines play a potentially important role in alterations of mood and cognition.
Objectives
This study aimed to examine the neurobiological effects of catecholamine depletion on thyroid hormones by measuring endocrine and cerebral metabolic function in unmedicated subjects with remitted major depressive disorder (RMDD) and in healthy controls.
Methods
This was a randomized, placebo-controlled, and double-blind crossover trial that included 15 unmedicated RMDD subjects and 13 healthy control subjects. The participants underwent two 3-day-long sessions at 1-week intervals; each participant was randomly administered oral α-methyl-para-tyrosine in one session (catecholamine depletion) and an identical capsule containing hydrous lactose (sham depletion) in the other session prior to a [18F]-fluorodeoxyglucose positron emission tomography scan.
Results
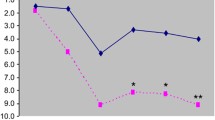
Serum concentrations of free T3 (FT3), free T4 (FT4), and TSH were obtained and assessed with respect to their relationship to regional cerebral glucose metabolism. Both serum FT3 (P = 0.002) and FT4 (P = 0.0009) levels were less suppressed after catecholamine depletion compared with placebo treatment in the entire study sample. There was a positive association between both FT3 (P = 0.0005) and FT4 (P = 0.002) and depressive symptoms measured using the Montgomery–Åsberg Depression Rating Scale. The relative elevation in FT3 level was correlated with a decrease in regional glucose metabolism in the right dorsolateral prefrontal cortex (rDLPFC; P < 0.05, corrected).
Conclusions
This study provided evidence of an association between a thyroid–catecholamine interaction and mood regulation in the rDLPFC.


Similar content being viewed by others
References
Altshuler LL, Bauer M, Frye MA, Gitlin MJ, Mintz J, Szuba MP, Leight KL, Whybrow PC (2001) Does thyroid supplementation accelerate tricyclic antidepressant response? A review and meta-analysis of the literature. Am J Psychiatry 158:1617–1622
Aronson R, Offman HJ, Joffe RT, Naylor CD (1996) Triiodothyronine augmentation in the treatment of refractory depression. A meta-analysis. Arch Gen Psychiatry 53:842–848
Bauer MS, Whybrow PC (1988) Thyroid hormones and the central nervous system in affective illness: interactions that may have clinical significance. Integr Psychiatry 6:75–85
Bauer M, Whybrow PC (2001) Thyroid hormone, neural tissue and mood modulation. World J Biol Psychiatry 2:59–69
Bauer M, Heinz A, Whybrow PC (2002) Thyroid hormones, serotonin and mood: of synergy and significance in the adult brain. Mol Psychiatry 7:140–156
Bauer M, London ED, Rasgon N, Berman SM, Frye MA, Altshuler LL, Mandelkern MA, Bramen J, Voytek B, Woods R, Mazziotta JC, Whybrow PC (2005) Supraphysiological doses of levothyroxine alter regional cerebral metabolism and improve mood in bipolar depression. Mol Psychiatry 10:456–469
Bauer M, Goetz T, Glenn T, Whybrow PC (2008) The thyroid–brain interaction in thyroid disorders and mood disorders. J Neuroendocrinol 20:1101–1114
Bauer M, Silverman DH, Schlagenhauf F, London ED, Geist CL, van Herle K, Rasgon N, Martinez D, Miller K, van Herle A, Berman SM, Phelps ME, Whybrow PC (2009) Brain glucose metabolism in hypothyroidism: a positron emission tomography study before and after thyroid hormone replacement therapy. J Clin Endocrinol Metab 94:2922–2929
Berman RM, Narasimhan M, Miller HL, Anand A, Cappiello A, Oren DA, Heninger GR, Charney DS (1999) Transient depressive relapse induced by catecholamine depletion: potential phenotypic vulnerability marker? Arch Gen Psychiatry 56:395–403
Buchanan J, Buchanan WW, Crooks J, Gale GE (1959) The use of reserpine in the treatment of thyrotoxicosis. Scott Med J 4:486–490
Canary JJ, Schaaf M, Duffy BJ Jr, Kyle LH (1957) Effects of oral and intramuscular administration of reserpine in thyrotoxicosis. N Engl J Med 257:435–442
Constant EL, de Volder AG, Ivanoiu A, Bol A, Labar D, Seghers A, Cosnard G, Melin J, Daumerie C (2001) Cerebral blood flow and glucose metabolism in hypothyroidism: a positron emission tomography study. J Clin Endocrinol Metab 86:3864–3870
Forchetti CM, Katsamakis G, Garron DC (1997) Autoimmune thyroiditis and a rapidly progressive dementia: global hypoperfusion on SPECT scanning suggests a possible mechanism. Neurology 49:623–626
Gershon AA, Dannon PN, Grunhaus L (2003) Transcranial magnetic stimulation in the treatment of depression. Am J Psychiatry 160:835–845
Gordon JT, Kaminski DM, Rozanov CB, Dratman MB (1999) Evidence that 3,3′,5-triiodothyronine is concentrated in and delivered from the locus coeruleus to its noradrenergic targets via anterograde axonal transport. Neuroscience 93:943–954
Harrison TS (1964) Adrenal medullary and thyroid relationships. Physiol Rev 44:161–185
Hasler G, Fromm S, Carlson PJ, Luckenbaugh DA, Waldeck T, Geraci M, Roiser JP, Neumeister A, Meyers N, Charney DS, Drevets WC (2008) Neural response to catecholamine depletion in unmedicated subjects with major depressive disorder in remission and healthy subjects. Arch Gen Psychiatry 65:521–531
Hasler G, Luckenbaugh DA, Snow J, Meyers N, Waldeck T, Geraci M, Roiser J, Knutson B, Charney DS, Drevets WC (2009) Reward processing after catecholamine depletion in unmedicated, remitted subjects with major depressive disorder. Biol Psychiatry 66:201–205
Hendrick V, Altshuler L, Whybrow P (1998) Psychoneuroendocrinology of mood disorders. The hypothalamic-pituitary-thyroid axis. Psychiatr Clin North Am 21:277–292
Homan P, Kindler J, Hubl D, Dierks T (2012) Auditory verbal hallucinations: imaging, analysis, and intervention. Eur Arch Psychiatry Clin Neurosci 262(Suppl 2):91–95
Joffe R (1993) The thyroid axis and psychiatric illness. American Psychiatric, Washington DC
Joffe RT (2006) Is the thyroid still important in major depression? J Psychiatry Neurosci 31:367–368
Joffe RT, Singer W (1990) A comparison of triiodothyronine and thyroxine in the potentiation of tricyclic antidepressants. Psychiatry Res 32:241–251
Joffe RT, Sokolov ST (1994) Thyroid hormones, the brain, and affective disorders. Crit Rev Neurobiol 8:45–63
Joffe R, Segal Z, Singer W (1996) Change in thyroid hormone levels following response to cognitive therapy for major depression. Am J Psychiatry 153:411–413
Kaptein EM, Spencer CA, Kamiel MB, Nicoloff JT (1980) Prolonged dopamine administration and thyroid hormone economy in normal and critically ill subjects. J Clin Endocrinol Metab 51:387–393
Kathol RG, Delahunt JW (1986) The relationship of anxiety and depression to symptoms of hyperthyroidism using operational criteria. Gen Hosp Psychiatry 8:23–28
Kinuya S, Michigishi T, Tonami N, Aburano T, Tsuji S, Hashimoto T (1999) Reversible cerebral hypoperfusion observed with Tc-99m HMPAO SPECT in reversible dementia caused by hypothyroidism. Clin Nucl Med 24:666–668
Krausz Y, Freedman N, Lester H, Newman JP, Barkai G, Bocher M, Chisin R, Bonne O (2004) Regional cerebral blood flow in patients with mild hypothyroidism. J Nucl Med 45:1712–1715
Krausz Y, Freedman N, Lester H, Barkai G, Levin T, Bocher M, Chisin R, Lerer B, Bonne O (2007) Brain SPECT study of common ground between hypothyroidism and depression. Int J Neuropsychopharmacol 10:99–106
Maayan ML (1990) Catecholamines and the thyroid. Thyroid 1:39–42
Mayberg HS (2003) Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull 65:193–207
Meyer T, Hesch RD (1983) Triiodothyronamine—a beta-adrenergic metabolite of triiodothyronine? Horm Metab Res 15:602–606
Meyers N, Fromm S, Luckenbaugh DA, Drevets WC, Hasler G (2011) Neural correlates of sleepiness induced by catecholamine depletion. Psychiatry Res 194:73–78
Moore DF, Altarescu G, Barker WC, Patronas NJ, Herscovitch P, Schiffmann R (2003) White matter lesions in Fabry disease occur in ‘prior’ selectively hypometabolic and hyperperfused brain regions. Brain Res Bull 62:231–240
Nagamachi S, Jinnouchi S, Nishii R, Ishida Y, Fujita S, Futami S, Kodama T, Tamura S, Kawai K (2004) Cerebral blood flow abnormalities induced by transient hypothyroidism after thyroidectomy—analysis by tc-99m-HMPAO and SPM96. Ann Nucl Med 18:469–477
Nagatsu T, Levitt M, Udenfriend S (1964) Tyrosine hydroxylase. The initial step in norepinephrine biosynthesis. J Biol Chem 239:2910–2917
Oda Y, Tajima K, Mori-Tanaka M, Matsui I, Kitajima K, Miyagawa J, Hanafusa T, Mashita K, Tarui S (1991) Alpha 1-adrenergic regulation of thyrotropin-stimulated release of 3,5,3′-triiodothyronine and thyroxine from perifused mouse thyroid. J Endocrinol Invest 14:867–873
Plosker SM, Rabinovici J, Montalvo M, Jaffe RB (1995) Endogenous catecholamines suppress thyrotropin secretion during the early follicular phase of the menstrual cycle. J Clin Endocrinol Metab 80:2530–2533
Ramadan W, Marsili A, Larsen PR, Zavacki AM, Silva JE (2011) Type-2 iodothyronine 5'deiodinase (D2) in skeletal muscle of C57Bl/6 mice. II. Evidence for a role of D2 in the hypermetabolism of thyroid hormone receptor alpha-deficient mice. Endocrinology 152:3093–102
Ruel J, Faure R, Dussault JH (1985) Regional distribution of nuclear T3 receptors in rat brain and evidence for preferential localization in neurons. J Endocrinol Invest 8:343–348
Schraml FV, Beason-Held LL, Fletcher DW, Brown BP (2006) Cerebral accumulation of Tc-99m ethyl cysteinate dimer (ECD) in severe, transient hypothyroidism. J Cereb Blood Flow Metab 26:321–329
Schueler PA, Schwartz HL, Strait KA, Mariash CN, Oppenheimer JH (1990) Binding of 3,5,3′-triiodothyronine (T3) and its analogs to the in vitro translational products of c-erbA protooncogenes: differences in the affinity of the alpha- and beta-forms for the acetic acid analog and failure of the human testis and kidney alpha-2 products to bind T3. Mol Endocrinol 4:227–234
Schwartz HL, Oppenheimer JH (1978) Nuclear triiodothyronine receptor sites in brain: probable identity with hepatic receptors and regional distribution. Endocrinology 103:267–273
Silva JE, Larsen PR (1983) Adrenergic activation of triiodothyronine production in brown adipose tissue. Nature 305:712–3
Stine SM, Krystal JH, Petrakis IL, Jatlow PI, Heninger GR, Kosten TR, Charney DS (1997) Effect of alpha-methyl-para-tyrosine on response to cocaine challenge. Biol Psychiatry 42:181–190
Verhoeff NP, Christensen BK, Hussey D, Lee M, Papatheodorou G, Kopala L, Rui Q, Zipursky RB, Kapur S (2003) Effects of catecholamine depletion on D2 receptor binding, mood, and attentiveness in humans: a replication study. Pharmacol Biochem Behav 74:425–432
Waldstein SS (1966) Thyroid–catecholamine interrelations. Annu Rev Med 17:123–132
Whybrow PC, Prange AJ Jr (1981) A hypothesis of thyroid–catecholamine–receptor interaction. Its relevance to affective illness. Arch Gen Psychiatry 38:106–113
Yamada M, Wilber JF (1990) Reciprocal regulation of preprothyrotropin-releasing hormone (TRH) mRNA in the rat anterior hypothalamus by thyroid hormone: dissociation from TRH concentrations during hypothyroidism. Neuropeptides 15:49–53
Zhu DF, Wang ZX, Zhang DR, Pan ZL, He S, Hu XP, Chen XC, Zhou JN (2006) fMRI revealed neural substrate for reversible working memory dysfunction in subclinical hypothyroidism. Brain 129:2923–2930
Zimmermann RC, Krahn LE, Klee GG, Ditkoff EC, Ory SJ, Sauer MV (2001) Prolonged inhibition of presynaptic catecholamine synthesis with alpha-methyl-para-tyrosine attenuates the circadian rhythm of human TSH secretion. J Soc Gynecol Investig 8:174–178
Acknowledgements
We are grateful to Nicolas Rodondi for his valuable comments on an earlier version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Homan, P., Drevets, W.C. & Hasler, G. Neural correlates of free T3 alteration after catecholamine depletion in subjects with remitted major depressive disorder and in controls. Psychopharmacology 231, 409–417 (2014). https://doi.org/10.1007/s00213-013-3250-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-3250-2