Abstract
Rationale
We recently demonstrated that blocking specific nicotinic acetylcholine receptors (nAChRs) abolishes the conditioned reinforcing properties of ethanol-associated cues in rat, suggesting nAChRs as promising pharmacological targets for prevention of cue-induced relapse.
Objectives
The present study investigated the involvement of nAChR subtypes in the conditioned reinforcing properties of stimuli associated with a natural reward (sucrose).
Methods
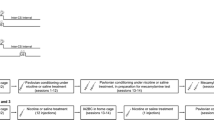
Water-deprived rats were trained to associate a tone + light stimulus (CS) with the presentation of a 0.1 M sucrose solution for 10 consecutive days. On the subsequent day, the animals were tested on the stringent acquisition of a new instrumental response with conditioned reinforcement, following a systemic injection of the nonselective nAChR antagonist mecamylamine (MEC) or the selective α7 and α6/α3β2β3* nAChR antagonist methyllycaconitine (MLA). At testing, the rats were presented with two novel levers. Responding on the lever assigned as active (CR lever) resulted in a presentation of the CS alone, while pressing the inactive lever (NCR lever) had no programmed consequences.
Results
Control animals pressed the CR lever significantly more than the NCR lever, demonstrating that the CR had acquired conditioned reinforcing properties. Systemic MEC as well as MLA reduced the CR lever responses to the same level as for the NCR lever.
Conclusions
These results demonstrate a role for the α7 and/or α6/α3β2β3* nAChRs in conditioned reinforcement to a natural reward and suggest neuronal nAChRs as common mediators of the impact of cues on incentive processes.




Similar content being viewed by others
Abbreviations
- nAChR:
-
Nicotinic acetylcholine receptor
- CS:
-
Conditioned stimulus
- US:
-
Unconditioned stimulus
- MEC:
-
Mecamylamine
- MLA:
-
Methyllycaconitine citrate
- CR:
-
Conditioned reinforcement
- VTA:
-
Ventral tegmental area
- PBS:
-
Phosphate-buffered saline
- VR:
-
Variable ratio
- ANOVA:
-
Analysis of variance
- PLSD:
-
Protected least-significant difference
- α-CtxMII:
-
α-Conotoxin MII
References
Alkondon M, Albuquerque EX (1993) Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. J Pharmacol Exp Ther 265:1455–1473
Alkondon M, Pereira EF, Wonnacott S, Albuquerque EX (1992) Blockade of nicotinic currents in hippocampal neurons defines methyllycaconitine as a potent and specific receptor antagonist. Mol Pharmacol 41:802–808
Brown J, Bullock D, Grossberg S (1999) How the basal ganglia use parallel excitatory and inhibitory learning pathways to selectively respond to unexpected rewarding cues. J Neurosci 19:10502–10511
Brunzell DH, Chang JR, Schneider B, Olausson P, Taylor JR, Picciotto MR (2005) Beta2-subunit-containing nicotinic acetylcholine receptors are involved in nicotine-induced increases in conditioned reinforcement but not progressive ratio responding for food in C57BL/6 mice. Psychopharmacology (Berl) 184:328–338
Burns LH, Everitt BJ, Kelley AE, Robbins TW (1994) Glutamate–dopamine interactions in the ventral striatum: role in locomotor activity and responding with conditioned reinforcement. Psychopharmacology (Berl) 115:516–528
Cardinal RN, Parkinson JA, Hall J, Everitt BJ (2002) Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev 26:321–352
Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Lena C, Clementi F, Moretti M, Rossi FM, Le Novere N, McIntosh JM, Gardier AM, Changeux JP (2003) Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci 23:7820–7829
Charpantier E, Barneoud P, Moser P, Besnard F, Sgard F (1998) Nicotinic acetylcholine subunit mRNA expression in dopaminergic neurons of the rat substantia nigra and ventral tegmental area. NeuroReport 9:3097–3101
Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Palmatier MI, Liu X, Sved AF (2006a) Operant responding for conditioned and unconditioned reinforcers in rats is differentially enhanced by the primary reinforcing and reinforcement-enhancing effects of nicotine. Psychopharmacology (Berl) 189:27–36
Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF (2006b) Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl) 184:353–366
Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O'Brien CP (1993) Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr 137:73–95
Corrigall WA, Coen KM, Adamson KL (1994) Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res 653:278–284
Davies AR, Hardick DJ, Blagbrough IS, Potter BV, Wolstenholme AJ, Wonnacott S (1999) Characterisation of the binding of [3H]methyllycaconitine: a new radioligand for labelling alpha 7-type neuronal nicotinic acetylcholine receptors. Neuropharmacology 38:679–690
Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF (2003) Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 169:68–76
Dormont JF, Conde H, Farin D (1998) The role of the pedunculopontine tegmental nucleus in relation to conditioned motor performance in the cat. I. Context-dependent and reinforcement-related single unit activity. Exp Brain Res 121:401–410
Ericson M, Molander A, Löf E, Engel JA, Söderpalm B (2003) Ethanol elevates accumbal dopamine levels via indirect activation of ventral tegmental nicotinic acetylcholine receptors. Eur J Pharmacol 467:85–93
Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW (1999) Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. Ann N Y Acad Sci 877:412–438
Grimm JW, Hope BT, Wise RA, Shaham Y (2001) Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature 412:141–142
Grunberg NE (1982) The effects of nicotine and cigarette smoking on food consumption and taste preferences. Addict Behav 7:317–331
Grunberg NE, Bowen DJ, Maycock VA, Nespor SM (1985) The importance of sweet taste and caloric content in the effects of nicotine on specific food consumption. Psychopharmacology (Berl) 87:198–203
Grunberg NE, Popp KA, Bowen DJ, Nespor SM, Winders SE, Eury SE (1988a) Effects of chronic nicotine administration on insulin, glucose, epinephrine, and norepinephrine. Life Sci 42:161–170
Grunberg NE, Popp KA, Winders SE (1988b) Effects of nicotine on body weight in rats with access to “junk” foods. Psychopharmacology (Berl) 94:536–539
Hall SM, McGee R, Tunstall C, Duffy J, Benowitz N (1989) Changes in food intake and activity after quitting smoking. J Consult Clin Psychol 57:81–86
Hatsukami DK, Hughes JR, Pickens RW, Svikis D (1984) Tobacco withdrawal symptoms: an experimental analysis. Psychopharmacology (Berl) 84:231–236
Hatsukami D, LaBounty L, Hughes J, Laine D (1993) Effects of tobacco abstinence on food intake among cigarette smokers. Health Psychol 12:499–502
Holland PC, Petrovich GD, Gallagher M (2002) The effects of amygdala lesions on conditioned stimulus-potentiated eating in rats. Physiol Behav 76:117–129
Inglis WL, Dunbar JS, Winn P (1994) Outflow from the nucleus accumbens to the pedunculopontine tegmental nucleus: a dissociation between locomotor activity and the acquisition of responding for conditioned reinforcement stimulated by d-amphetamine. Neuroscience 62:51–64
Inglis WL, Olmstead MC, Robbins TW (2000) Pedunculopontine tegmental nucleus lesions impair stimulus–reward learning in autoshaping and conditioned reinforcement paradigms. Behav Neurosci 114:285–294
Jensen AA, Frolund B, Liljefors T, Krogsgaard-Larsen P (2005) Neuronal nicotinic acetylcholine receptors: structural revelations, target identifications, and therapeutic inspirations. J Med Chem 48:4705–4745
Junghanns K, Veltrup C, Wetterling T (2000) Craving shift in chronic alcoholics. Eur Addict Res 6:64–70
Kampov-Polevoy AB, Garbutt JC, Janowsky DS (1999) Association between preference for sweets and excessive alcohol intake: a review of animal and human studies. Alcohol Alcohol 34:386–395
Kelley AE, Berridge KC (2002) The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci 22:3306–3311
Klink R, de Kerchove d'Exaerde A, Zoli M, Changeux JP (2001) Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci 21:1452–1463
Löf E, Olausson P, de Bejczy A, Stomberg R, McIntosh J, Taylor J, Söderpalm B (2007) Nicotinic acetylcholine receptors in the ventral tegmental area mediate the dopamine activating and reinforcing properties of ethanol cues. Psychopharmacology (Berl) 195:333–343
Macallan DRE, Lunt GG, Wonnacott S, Swanson KL, Rapoport H, Albuquerque EX (1988) Methyllycaconitine and (+)-anatoxin-a differentiate between nicotinic receptors in vertebrate and invertebrate nervous systems. FEBS Lett 226:357–363
Mameli-Engvall M, Evrard A, Pons S, Maskos U, Svensson TH, Changeux JP, Faure P (2006) Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron 50:911–921
Miller AD, Forster GL, Metcalf KM, Blaha CD (2002) Excitotoxic lesions of the pedunculopontine differentially mediate morphine- and d-amphetamine-evoked striatal dopamine efflux and behaviors. Neuroscience 111:351–362
Mogg AJ, Whiteaker P, McIntosh JM, Marks M, Collins AC, Wonnacott S (2002) Methyllycaconitine is a potent antagonist of alpha-conotoxin-MII-sensitive presynaptic nicotinic acetylcholine receptors in rat striatum. J Pharmacol Exp Ther 302:197–204
Mugnaini M, Tessari M, Tarter G, Merlo Pich E, Chiamulera C, Bunnemann B (2002) Upregulation of [3H]methyllycaconitine binding sites following continuous infusion of nicotine, without changes of alpha7 or alpha6 subunit mRNA: an autoradiography and in situ hybridization study in rat brain. Eur J Neurosci 16:1633–1646
Nie H, Janak PH (2003) Comparison of reinstatement of ethanol- and sucrose-seeking by conditioned stimuli and priming injections of allopregnanolone after extinction in rats. Psychopharmacology 168:222–228
O'Brien CP, Childress AR, Ehrman R, Robbins SJ (1998) Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol 12:15–22
O'Dell TJ, Christensen BN (1988) Mecamylamine is a selective non-competitive antagonist of N-methyl-d-aspartate- and aspartate-induced currents in horizontal cells dissociated from the catfish retina. Neurosci Lett 94:93–98
Olausson P, Jentsch JD, Taylor JR (2004a) Nicotine enhances responding with conditioned reinforcement. Psychopharmacology (Berl) 171:173–178
Olausson P, Jentsch JD, Taylor JR (2004b) Repeated nicotine exposure enhances responding with conditioned reinforcement. Psychopharmacology (Berl) 173:98–104
Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, Liu X, Booth S, Gharib M, Craven L, Sved AF (2006) Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology (Berl) 184:391–400
Pan WX, Hyland BI (2005) Pedunculopontine tegmental nucleus controls conditioned responses of midbrain dopamine neurons in behaving rats. J Neurosci 25:4725–4732
Passilta M, Kervinen K, Kesaniemi YA (1999) Glucose metabolism, insulin-like growth factor-I, and insulin-like growth factor-binding protein-1 after alcohol withdrawal. Alcohol Clin Exp Res 23:471–475
Petrovich GD, Setlow B, Holland PC, Gallagher M (2002) Amygdalo-hypothalamic circuit allows learned cues to override satiety and promote eating. J Neurosci 22:8748–8753
Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP (1998) Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 391:173–177
Reid MS, Mickalian JD, Delucchi KL, Hall SM, Berger SP (1998) An acute dose of nicotine enhances cue-induced cocaine craving. Drug Alcohol Depend 49:95–104
Reid MS, Mickalian JD, Delucchi KL, Berger SP (1999) A nicotine antagonist, mecamylamine, reduces cue-induced cocaine craving in cocaine-dependent subjects. Neuropsychopharmacology 20:297–307
Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM (2004) Dopamine operates as a subsecond modulator of food seeking. J Neurosci 24:1265–1271
Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, Grady SR (2004) Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol 65:1526–1535
Schultz W (1998) Predictive reward signal of dopamine neurons. J Neurophysiol 80:1–27
See RE (2002) Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav 71:517–529
Snell LD, Johnson KM (1989) Effects of nicotinic agonists and antagonists on N-methyl-d-aspartate-induced 3H-norepinephrine release and 3H-(1-[1-(2-thienyl)cyclohexyl]-piperidine) binding in rat hippocampus. Synapse 3:129–135
Taylor JR, Horger BA (1999) Enhanced responding for conditioned reward produced by intra-accumbens amphetamine is potentiated after cocaine sensitization. Psychopharmacology (Berl) 142:31–40
Taylor JR, Robbins TW (1984) Enhanced behavioural control by conditioned reinforcers following microinjections of d-amphetamine into the nucleus accumbens. Psychopharmacology (Berl) 84:405–412
Turek JW, Kang CH, Campbell JE, Arneric SP, Sullivan JP (1995) A sensitive technique for the detection of the alpha 7 neuronal nicotinic acetylcholine receptor antagonist, methyllycaconitine, in rat plasma and brain. J Neurosci Methods 61:113–118
Ward JM, Cockcroft VB, Lunt GG, Smillie FS, Wonnacott S (1990) Methyllycaconitine: a selective probe for neuronal [alpha]-bungarotoxin binding sites. FEBS Lett 270:45–48
Weingarten HP (1983) Conditioned cues elicit feeding in sated rats: a role for learning in meal initiation. Science 220:431–433
Yu CR, Role LW (1998) Functional contribution of the alpha7 subunit to multiple subtypes of nicotinic receptors in embryonic chick sympathetic neurones. J Physiol 509(Pt 3):651–665
Yun IA, Wakabayashi KT, Fields HL, Nicola SM (2004) The ventral tegmental area is required for the behavioral and nucleus accumbens neuronal firing responses to incentive cues. J Neurosci 24:2923–2933
Acknowledgements
Financial support for this work was obtained from the Swedish Medical Research Council no. 11583, the Swedish Labor Market Insurance (AFA) support for biomedical alcohol research, the Alcohol Research Council of the Swedish Alcohol Retailing Monopoly, NIDA 2 R01 10765-04A1, PHS NIH (DA15222, DA11717, and AA15632 to JRT), Gunnar och Märtha Bergendahls Stiftelse, the Council for Medical Tobacco Research - Swedish Match, Wilhelm och Martina Lundgrens vetenskapsfond, Kungliga Vetenskaps -och Vitterhets-Samhället i Göteborg, Helge Ax:son Johnsons Stiftelse, Längmanska kulturfonden, Jubileumsfonden, Iris Jonzén-Sandbloms och Greta Jonzéns Stiftelse and Stiftelsen KvinnorKan, Axel Linders stiftelse, and Apotekarsocieteten. We are grateful for the generous gift of mecamylamine and MLA from the NIDA drug supply program, Bethesda, MD, USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Löf, E., Olausson, P., Stomberg, R. et al. Nicotinic acetylcholine receptors are required for the conditioned reinforcing properties of sucrose-associated cues. Psychopharmacology 212, 321–328 (2010). https://doi.org/10.1007/s00213-010-1957-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-010-1957-x