Abstract
Rationale
Converging lines of evidence suggest an association between cannabis use and impaired episodic memory as well as related associative learning. These deficits have been associated with the duration, frequency, and age of onset of cannabis use. However, it remains unclear whether these parameters of use differently impact memory-related hippocampal functioning.
Methods
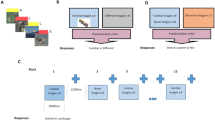
Forty-two cannabis users were examined by means of functional magnetic resonance imaging while they encoded and retrieved face–profession associations. Region of interest analysis was subsequently used to compare (para-)hippocampal functioning in users with (1) a longer and shorter duration of use, (2) a higher and lower frequency of use, and (3) an earlier and later onset. To further separate the effects of these parameters of use on performance and (para-)hippocampal activity, linear regression analysis was applied.
Results
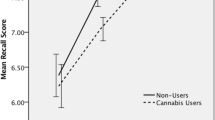
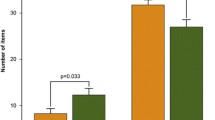
Compared to low-frequency users, high-frequency users displayed stronger blood oxygenation level-dependent response during encoding in the left parahippocampal gyrus. No differences were obvious for the groups separated according to duration of use or an earlier and later onset of use. Linear regression analysis confirmed the association between a higher frequency of use and increased activity in the left parahippocampal gyrus.
Conclusions
Our findings suggest that the frequency of use might have a particular critical impact on intact parahippocampal functioning in cannabis users. Increased activity within the encoding-related network might reflect functional compensation to maintain cognitive functioning.



Similar content being viewed by others
References
Block RI, Wittenborn JR (1985) Marijuana effects on associative processes. Psychopharmacology 85:426–430
Block RI, O'Leary DS, Ehrhardt JC, Augustinack JC, Ghoneim MM, Arndt S, Hall JA (2000) Effects of frequent marijuana use on brain tissue volume and composition. NeuroReport 11:491–496
Block RI, O'Leary DS, Hichwa RD, Augustinack JC, Boles Ponto LL, Ghoneim MM, Arndt S, Hurtig RR, Watkins GL, Hall JA, Nathan PE, Andreasen NC (2002) Effects of frequent marijuana use on memory-related regional cerebral blood flow. Pharmacol Biochem Behav 72:237–250
Bondi MW, Houston WS, Eyler LT, Brown GG (2005) fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology 64:501–508
Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW (2000) Patterns of brain activation in people at risk for Alzheimer's disease. N Engl J Med 343:450–456
Callicott JH, Bertolino A, Mattay VS, Langheim FJP, Duyn J, Coppola R, Goldberg TE, Weinberger DR (2000) Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex 10:1078–1092
Chang L, Speck O, Miller EN, Braun J, Jovicich J, Koch C, Itti L, Ernst T (2001) Neural correlates of attention and working memory deficits in HIV patients. Neurology 57:1001–1007
Chang L, Yakupov R, Cloak C, Ernst T (2006) Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain 129:1096–1112
Daumann J, Fischermann T, Heekeren K, Thron A, Gouzoulis-Mayfrank E (2004) Neural mechanisms of working memory in ecstasy (MDMA) users who continue or discontinue ecstasy and amphetamine use: evidence from an 18-month longitudinal functional magnetic resonance imaging study. Biol Psychiatry 56:349–355
Daumann J, Fischermann T, Heekeren K, Henke K, Thron A, Gouzoulis-Mayfrank E (2005) Memory-related hippocampal dysfunction in poly-drug ecstasy (3, 4-methylenedioxymethamphetamine) users. Psychopharmacology 180:607–611
Davachi L, Wagner AD (2002) Hippocampal contributions to episodic encoding: insights from relational and item-based learning. J Neurophysiol 88:982–990
Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, Gigerenzer G, Hoehe MR (1999) Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology 142:295–301
Eldreth DA, Matochik JA, Cadet JL, Bolla KI (2004) Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. NeuroImage 23:914–920
Fried PA, Watkinson B, Gray R (2005) Neurocognitive consequences of marihuana—a comparison with pre-drug performance. Neurotoxicol Teratol 27:231–239
Grant I, Gonzalez R, Carey CL, Natarajan L, Wolfson T (2003) Non-acute (residual) neurocognitive effects of cannabis use: a meta-analytic study. J Int Neuropsychol Soc 9:679–689
Harvey MA, Sellman JD, Porter RJ, Frampton CM (2007) The relationship between non-acute adolescent cannabis use and cognition. Drug Alcohol Rev 26:309–319
Henke K, Buck A, Weber B, Wieser HG (1997) Human hippocampus establishes associations in memory. Hippocampus 7:249–256
Henke K, Weber B, Kneifel S, Wieser HG, Buck A (1999) Human hippocampus associates information in memory. Proc Natl Acad Sci USA 96:5584–5589
Henke K, Treyer V, Nagy ET, Kneifel S, Dürsteler M, Nitsch RM, Buck A (2003) Active hippocampus during nonconscious memories. Conscious Cogn 12(1):31–48
Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC (1991) Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci 11:563–583
Herning RI, Better WE, Tate K, Cadet JL (2005) Cerebrovascular perfusion in marijuana users during a month of monitored abstinence. Neurology 64:488–493
Hoffman AF, Oz M, Yang R, Lichtman AH, Lupica CR (2007) Opposing actions of chronic delta-9-tetrahydrocannabinol and cannabinoid antagonists on hippocampal long-term potentiation. Learn Mem 14:63–74
Indlekofer F, Piechatzek M, Daamen M, Glasmacher C, Lieb R, Pfister H, Tucha O, Lange KW, Wittchen HU, Schütz CG (2008) Reduced memory and attention performance in a population-based sample of young adults with a moderate lifetime use of cannabis, ecstasy and alcohol. J Psychopharmacol 23:495–509
Jacobsen LK, Pugh KR, Constable RT, Westerveld M, Mencl WE (2007) Functional correlates of verbal memory deficits emerging during nicotine withdrawal in abstinent adolescent cannabis users. Biol Psychiatry 61:31–40
Jager G, Van Hell HH, De Win MM, Kahn RS, Van Den Brink W, Van Ree JM, Ramsey NF (2007) Effects of frequent cannabis use on hippocampal activity during an associative memory task. Eur Neuropsychopharmacol 17:289–297
Kanayama G, Rogowska J, Pope HG, Gruber SA, Yurgelun-Todd DA (2004) Spatial working memory in heavy cannabis users: a functional magnetic resonance imaging study. Psychopharmacology 176:239–247
Kirwan CB, Stark CE (2004) Medial temporal lobe activation during encoding and retrieval of novel face-name pairs. Hippocampus 14:919–930
Landfield PW, Cadwallader LB, Vinsant S (1988) Quantitative changes in hippocampal structure following long-term exposure to delta 9-tetrahydrocannabinol: possible mediation by glucocorticoid systems. Brain Res 443:47–62
Lawston J, Borella A, Robinson JK, Whitaker-Azmitia PM (2000) Changes in hippocampal morphology following chronic treatment with the synthetic cannabinoid WIN 55, 212–2. Brain Res 877:407–410
Lichtman AH, Dimen KR, Martin BR (1995) Systemic or intrahippocampal cannabinoid administration impairs spatial memory in rats. Psychopharmacology (Berl) 119:282–290
Maldjian JA, Laurienti PJ, Burdette JB, Kraft RA (2003) An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage 19:1233–1239
Maldjian JA, Laurienti PJ, Burdette JH (2004) Precentral gyrus discrepancy in electronic versions of the talairach atlas. NeuroImage 21:450–455
Martin GW, Wilkinson DA, Kapur BM (1988) Validation of self-reported cannabis use by urine analysis. Addict Behav 13:147–150
Matochik JA, Eldreth DA, Cadet JL, Bolla KI (2005) Altered brain tissue composition in heavy marijuana users. Drug Alcohol Depend 77:23–30
McGilveray IJ (2005) Pharmacokinetics of cannabinoids. Pain Res Manag 10:15–22
Melrose RJ, Tinaz S, Castelo JM, Courtney MG, Stern CE (2008) Compromised fronto-striatal functioning in HIV: an fMRI investigation of semantic event sequencing. Behav Brain Res 188:337–347
Messinis L, Kyprianidou A, Malefaki S, Papathanasopoulos P (2006) Neuropsychological deficits in long-term frequent cannabis users. Neurology 66(5):737–739
Misner DL, Sullivan JM (1999) Mechanism of cannabinoid effects on long-term potentiation and depression in hippocampal CA1 neurons. J Neurosci 19:6795–6805
Nestler E, Hyman SE, Malenka R (2001) Serotonin, acetylcholine, and histamine. In: Nestler EJ, Hyman SE, Malenka RC (eds) Molecular neurophramacology: a foundation for clinical neuroscience. McGraw-Hill Co Inc., New York, pp 200–208
Nestor L, Roberts G, Garavan H, Hester R (2008) Deficits in learning and memory: parahippocampal hyperactivity and frontocortical hypoactivity in cannabis users. Neuroimage 40(3):1328–1339
O'Leary DS, Block RI, Koeppel JA, Flaum M, Schultz SK, Andreasen NC, Ponto LB, Watkins GL, Hurtig RR, Hichwa RD (2002) Effects of smoking marijuana on brain perfusion and cognition. Neuropsychopharmacology 26:802–816
Pope HG Jr, Yurgelun-Todd D (1996) The residual cognitive effects of heavy marijuana use in college students. JAMA 275(7):521–527
Pope HG, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D (2001) Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry 58:909–915
Pope HG Jr, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D (2003) Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend 69:303–310
Rothe M, Pragst F, Spiegel K, Harrach T, Fischer K, Kunkel J (1997) Hair concentrations and self-reported abuse history of 20 amphetamine and ecstasy users. Forensic Sci Int 89:111–128
Scallet AC, Uemura E, Andrews A, Ali SF, McMillan DE, Paule MG, Brown RM, Slikker W Jr (1987) Morphometric studies of the rat hippocampus following chronic delta-9-tetrahydrocannabidol (THC). Brain Res 436:193–198
Schacter DL, Wagner AD (1999) Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieva. Hippocampus 9:7–24
Schneider M (2008) Puberty as a highly vulnerable developmental period for the consequences of cannabis exposure. Addict Biol 13:253–263
Schweinsburg AD, Nagel BJ, Schweinsburg BC, Park A, Theilmann RJ, Tapert SF (2008) Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiat Res-Neuroimg 163:40–51
Sneider JT, Pope HG Jr, Silveri MM, Simpson NS, Gruber SA, Yurgelun-Todd DA (2006) Altered regional blood volume in chronic cannabis smokers. Exp Clin Psychopharmacol 14:422–428
Solowij N, Battisti R (2008) The chronic effects of cannabis on memory in humans: a review. Current Drug Abuse Reviews 1:81–98
Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, Christiansen K, McRee B, Vendetti J (2002) Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA 287:1123–1131
Sperling R, Chua E, Cocchiarella A, Rand-Giovannetti E, Poldrack R, Schacter DL, Albert M (2003) Putting names to faces: sucessful encoding of associative memories activates the anterior hippocampal formation. NeuroImage 20:1400–1410
Stella N, Schweitzer P, Piomelli D (1997) A second endogenous cannabinoid that modulates long-term potentiation. Nature 388:773–778
Tanabe J, Tregellas JR, Martin LF, Freedman R (2006) Effects of nicotine on hippocampal and cingulate activity during smooth pursuit eye movement in schizophrenia. Biol Psychiatry 59:754–761
Tsou K, Brown S, Sanudo-Pena MC, Mackie W, Walker JM (1998) Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 82:393–411
Tzilos GK, Cintron CB, Wood JB, Simpson NS, Young AD, Pope HG Jr, Yurgelun-Todd DA (2005) Lack of hippocampal volume change in long-term heavy cannabis users. Am J Addict 14:64–72
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 15:273–289
Ward MF, Wender PH, Reimherr FW (1993) The Wender Utah Rating Scale: an aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. Am J Psychiatry 150:885–890
Weniger G, Boucsein K, Irle E (2004) Impaired associative memory in temporal lobe epilepsy subjects after lesions of hippocampus, parahippocampal gyrus, and amygdala. Hippocampus 14:785–796
Xu J, Mendrek A, Cohen MS, Monterosso J, Rodriguez P, Simon SL, Brody A, Jarvik M, Domier CP, Olmstead R, Ernst M, London ED (2005) Brain activity in cigarette smokers performing a working memory task: effect of smoking abstinence. Biol Psychiatry 58:143–150
Yücel M, Solowij N, Respondek C, Whittle S, Fornito A, Pantelis C, Lubman DI (2008) Regional brain abnormalities associated with long-term heavy cannabis use. Arch Gen Psychiatry 65:694–701
Zeineh MM, Engel SA, Thompson PM, Bookheimer SY (2003) Dynamics of the hippocampus during encoding and retrieval of face-name pairs. Science 299:577–580
Acknowledgements
This work was supported by a grant to E. Gouzoulis-Mayfrank and J. Daumann from the Deutsche Forschungsgemeinschaft (DFG GO 717/6-1/2). We thank Ralf Wischnewski (ansprechbar/partypack.de) for help on recruiting volunteers.
Conflicts of interest
The authors have no conflicts of interest. The experiments comply with the current laws of Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Becker, B., Wagner, D., Gouzoulis-Mayfrank, E. et al. Altered parahippocampal functioning in cannabis users is related to the frequency of use. Psychopharmacology 209, 361–374 (2010). https://doi.org/10.1007/s00213-010-1805-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-010-1805-z