Abstract
Rationale
Dopamine (DA) plays an important role in working memory. However, the precise functions supported by different DA receptor subtypes in different neural regions remain unclear.
Objective
The present study used pharmacological, event-related fMRI to test the hypothesis that striatal dopamine is important for the manipulation of information in working memory.
Methods
Twenty healthy human subjects were scanned twice, once after placebo and once after sulpiride 400 mg, a selective DA D2 receptor antagonist, while performing a verbal working memory task requiring different levels of manipulation.
Results
Whilst there was no overall effect of sulpiride on task-dependent activation, individual variation in sulpiride plasma levels predicted the effect of working memory manipulation on activation in the putamen, suggesting a dose-dependent effect of DA antagonism on a striatally based manipulation process. These effects occurred in the context of a drug-induced improvement in performance on trials requiring the manipulation of information in working memory but not on simple retrieval trials. No significant drug effects were observed in the prefrontal cortex.
Conclusions
These results support models of dopamine function that posit a ‘gating’ function for dopamine D2 receptors in the striatum, which enables the flexible updating and manipulation of information in working memory.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well established that dopamine (DA) D1 receptors in the prefrontal cortex (PFC) are critical for the maintenance of task-relevant information in working memory. Performance of monkeys on delayed response tasks is impaired after DA depletion (Brozoski et al. 1979) and is specifically dependent on DA D1 receptor activity (Floresco et al. 2006).
DA in the striatum also plays an important role in working memory. In Parkinson’s disease (PD), progressive loss of DA neurons in the striatum leads to a profile of cognitive deficits that is qualitatively similar to that of patients with frontal lobe damage, which includes impairments in working memory and attentional flexibility (Owen 2004). However, the precise cognitive function supported by striatal DA remains uncertain.
Converging evidence from monkeys and humans suggests that DA exerts differential effects on cognition in the PFC and striatum. Crofts et al. (2001) found differential effects of striatal and prefrontal DA depletion on the acquisition of an attentional set in monkeys, with depletion of DA in the PFC leading to increased distractibility and depletion of DA in the striatum leading to more rigid responding. Dodds et al. (2008) investigated the effects of methylphenidate, a non-specific DA agonist, on activation during reversal learning in healthy human subjects and found that methylphenidate modulated striatal activation when subjects switched their response following negative feedback but prefrontal activation when subjects did not reverse following negative feedback. These studies suggest that striatal DA enables flexible responding while prefrontal DA enables the rigid or focused maintenance of responding.
There is also some evidence to suggest that working memory deficits associated with striatal DA loss in PD are subtly different from the deficits observed after DA depletion in the prefrontal cortex. Patients with PD are unimpaired when a working memory task requires the maintenance and subsequent retrieval of spatial information but are impaired on a task requiring active manipulation of that information (Owen et al. 1992; Lewis et al. 2003), suggesting that working memory deficits after striatal DA depletion are more severe in conditions requiring the updating and flexible manipulation of information held in working memory.
It has been hypothesised that regionally specific effects of DA reflect different functional roles for different DA receptor subtypes in working memory. Specifically, D1 receptor signalling in the PFC supports the stable maintenance of working memory representations, while D2 receptor signalling in the striatum acts as a ‘gating’ mechanism, facilitating or preventing the access of new working memory representations into the PFC (Braver et al. 1999; Frank et al. 2001). This hypothesis receives support from a recent functional neuroimaging study by Cools et al. (2007) who found that bromocriptine, a selective DA D2 agonist, modulated striatal activation during switching but lateral prefrontal activation during distraction in high-impulsive subjects.
Thus, functional neuroimaging evidence suggests that increasing DA function in the striatum modulates activation associated with the flexible updating of working memory representations, and that this process may be dependent on D2 receptor signalling. However, no study has, as yet, investigated the effects of selectively reducing DA D2 receptor signalling on event-related activation associated with different component processes of working memory. Mehta et al. (2003) used positron emission tomography (PET) to investigate the effects of sulpiride on striatal regional cerebral blood flow (rCBF) during a working memory task and found no effect of the drug on rCBF associated specifically with working memory. There was, however, some evidence that sulpiride attenuated rCBF in the caudate nucleus during a more complex planning task and that the drug-related changes in rCBF were associated with modulations of performance.
In the present study, we used event-related fMRI to investigate the effects of a single dose of sulpiride on activation during a working memory task requiring different levels of manipulation of information in working memory. This task has been shown previously to be sensitive to working memory deficits in PD (Bublak et al. 2002; Lewis et al. 2003) and to activate discrete prefrontal and striatal regions during fMRI in healthy volunteers (Lewis et al. 2004).
Participants were scanned once after placebo and once after sulpiride 400 mg while performing the working memory task, in the context of a larger design that also examined the effects of methylphenidate. We predicted that sulpiride would selectively affect subjects’ ability to manipulate information in working memory by modulating striatal activation specifically related to the process of manipulation, i.e. in the contrast of manipulation trials–simple retrieval trials. Furthermore, given that 400 mg of sulpiride has been shown to lead to only 28% occupancy of DA D2 receptors in the striatum (Mehta et al. 2008), we expected that effects of sulpiride on striatal activation would be sensitive to individual differences in drug absorption. We therefore predicted that sulpiride plasma levels would correlate with the magnitude of activation in the contrast of manipulation–simple retrieval trials.
Method
Participants
Twenty healthy, right-handed volunteers (mean age 22.2, range 19–33 years; 14 male and six female) took part in the study. Data from three participants were excluded from the final analysis due to problems with fMRI data acquisition. Mean verbal IQ measured with the National Adult Reading Test value was 121, range 116–126. Participants were recruited among students and staff of Cambridge University and Addenbrooke’s Hospital. All participants entered the study after screening by a research psychiatrist (UM) and had no major psychiatric, neurological or medical illness, including alcohol and drug abuse. They were asked to abstain from alcohol for 12 h, as well as from caffeine and nicotine for 3 h, before testing. A light breakfast or snack was allowed before, but not during, testing. All participants were questioned about compliance with alcohol and caffeine restrictions before inclusion into the study. All participants gave written informed consent prior to testing and received monetary compensation (£200). The protocol was approved by the Local Research Ethics Committee Cambridge (LREC No. 03/266) and exempted from clinical trial regulations by Medicines and Healthcare products Regulatory Agency.
Pharmacological design and procedures
The present study was carried out as part of a larger four-arm design which also investigated the effects of methylphenidate and sulpiride-methylphenidate combined. However, for clarity here we report only the results from the sulpiride vs placebo analysis.
The minimum time between testing sessions was 3 days. Single oral doses of sulpiride 400 mg (Dolmatil®, Sanofi-Synthelabo, Guildford, UK) or placebo (lactose with microcrystalline cellulose) contained in identical opaque gelatine capsules were administered 2 h before scanning. Between dosing and scanning, all volunteers were asked to spend the waiting time with low arousing activities (reading, watching TV etc.) in a day room and were monitored by research nurses. Dose selection was based on previous, similar studies showing behavioural effects at similar (and lower) doses using the same drugs in healthy volunteers (Mehta et al. 1999). The timing of scanning was performed around the time of maximal plasma level (Sugnaux et al. 1983; Wagstaff et al. 1994).
Prior to scanning on the first session, participants were trained on the task to reduce practise effects. If the gap between sessions was more than a week participants were re-trained on subsequent sessions.
Immediately before and after scanning, a blood sample was taken from each participant in order to measure drug plasma levels. Participants also completed visual analogue scales (VAS), which probed different aspects of participants’ current subjective experiences, at three time-points: baseline (prior to dosing), immediately before scanning and immediately after scanning (see Bond and Lader 1974, for details of the scales).
Unblinding was performed after the analysis of behavioural data and the first-level analysis of fMRI data.
Sulpiride plasma level analysis
Sulpiride plasma levels were analysed by liquid chromatography/mass spectrometry (Gschwend et al. 2006) using a Finnigan SSQ-7000 (Finnigan/MAT, Bremen, Germany, now Thermo Electron Corporation) mass spectrometer equipped with an electrospray ionisation (ESI)/APCI interface coupled with a ConstaMetric 4100 MS series pump, a SCM 1000 Vacuum Membran Degasser, an autosampler AS 3000, and a model 3200 programmable wavelength detector (Thermo Separation Products, Riviera Beach, Florida, USA) in ESI SIM mode by monitoring of the [M+H]+ ion of the analytes [m/z 342, 237 for sulpiride and D3-methylphenidate as an internal standard (ISTD)], respectively. Plasma samples, quality controls and calibration standards of each 0.5 ml were processed by solid phase extraction (Oasis HLB, Waters Corporation, Milford, USA). Chromatrographic separation was achieved on Ultrasep RP 18E column (150 × 2 mm, Sepserv, Berlin, Germany). A linear relationship between concentration and signal intensity (given as peak height ratio analyte/ISTD) was obtained (linear regression, 0.998) over detection range of the lower limit of quantification (LLQ) of 30 up to 1,500 ng/mL. The lower limit of detection was 6 ng/mL, recovery rate of sulpiride was 58%, accuracy and precision were 11% and 6%, respectively, and within- and between-day coefficients of variation were below 10%.
Working memory task
On each trial, subjects were presented sequentially with four consonants which they were required to hold in memory. The consonants were presented with a 1-s interval between each letter. After stimulus presentation, subjects saw a blank screen for between 9 and 14 s which served as a maintenance period. At the end of the maintenance period, a cue appeared which instructed subjects either to retrieve the letters from memory in the same order in which they were presented (retrieval condition), or to rearrange the letters into a different order (manipulation condition). In the manipulation condition subjects could be instructed either to recall the letters in the following order: third; fourth; first; second (‘pairs’ condition), or, alternatively in the following order: first; third; second; fourth (‘middle’ condition). For the retrieval condition, the cue was the word ‘same’, while for the manipulation condition the cue was either the word ‘middle’ or ‘pairs’. Following the cue, a blank screen was presented until subjects indicated that they had performed either the required manipulation process or the simple retrieval process. Subjects were instructed to ensure that they had performed these processes completely before they pressed the button. When the subject pressed the button, two letter strings were presented, one above the other, one of which was the ‘correct’ sequence, and the other an incorrect sequence. Subjects were required to indicate, by pressing one of two buttons under their index and middle fingers, which sequence was correct. This second response period extending from presentation of the choice array through to the second button press served as a motor control period for the motoric demands of the first response period. This second button press triggered a feedback message (1 s) indicating whether the choice was correct or incorrect. Subjects performed two blocks of 30 randomised trials of the working memory task, with an equal number of simple retrieval and manipulation trials (Fig. 1).
A single trial of the working memory task. Subjects retained four letters in working memory for a variable maintenance period. They were then cued to either (a) recall the letters in the same order in which they were initially presented or (b) reorder the letters into a prespecified order. After indicating with a button press that they were ready to respond (response 1), subjects were required to select the correct sequence from a two-choice array (response 2). Finally, feedback was given and there was a rest before the next trial began. In the example trial shown here the subject was cued to reorder the letters by swapping the locations of the first two letters and the last two letters (‘pairs’ cue). The subject then correctly selected the uppermost sequence from the choice array (indicated here by the white pointer which was not displayed in the actual task) and received correct feedback
Data acquisition and analysis
Participants were scanned at the Wolfson Brain Imaging Centre (University of Cambridge, UK) on a 3T Bruker scanner using a head coil. Functional images were collected using 21 slices covering the whole brain (slice thickness 4 mm, interslice gap 1 mm, in-plane resolution 1.56 × 1.56 mm) with an EPI sequence (TR, 1.6 s; TE 27 ms; matrix size, 128 × 128). The first 12 volumes were discarded to allow for T1-equilibrium effects. Structural and functional images were collected in the axial oblique plane.
All fMRI data were preprocessed (transformed) using SPM2 and analysed using SPM5 software (Wellcome Department of Cognitive Neurology, London). During preprocessing, all images were corrected for slice timing, subject motion corrected and geometrically undistorted using phase maps (Cusack et al. 2003). Using the mean realigned image, all images were coregistered to a skull-stripped (using the Brain Extraction Tool, Smith et al. 2002), high-resolution structural scan (voxel size, 1 × 1 × 1 mm) which was acquired on the first scanning day. Images were then normalised, using affine and smoothly nonlinear transformations, to an EPI template in Montreal Neurological Institute (MNI) space. Finally, all normalised images were spatially smoothed with a 10 mm full-width, half-maximum Gaussian kernel. The time-series were high-pass filtered (128 s) and a canonical haemodynamic response function was modelled to the onset of each event.
Statistical parametric maps were calculated with a general linear model (Friston et al. 1995). For each subject, separate first-level models were generated for the placebo and sulpiride sessions. This first-level analysis included covariates for neuronal responses elicited during stimulus presentation, maintenance period, cue (retrieval or manipulation), first motor response, second motor response and rest. A variable length boxcar was used to model all events except stimulus presentation, which was a fixed duration, and the second motor response, which was modelled as a delta function of zero duration.
Contrasts
In order to delineate the network of areas involved in performance of the task independently of any drug effects, we computed a contrast, cue minus maintenance period, at the subject-specific level, separately on scans from the placebo and sulpiride sessions, and then calculated the average of the two contrast images from the placebo and sulpiride sessions. The contrast images from this comparison were taken to a second-level analysis involving a one-sample t test in order to test for effects at the group level.
In order to examine whether any regions showed activation specifically related to manipulation, we computed a contrast, manipulation cue–simple retrieval cue, at the subject-specific level, separately on scans from the placebo and sulpiride sessions, and then calculated the average of the two contrast images from the placebo and sulpiride sessions. The contrast images from this comparison were taken to a second-level analysis involving a one-sample t test in order to test for effects at the group level.
Our prediction that drug effects on process-specific activity would be restricted to the striatum motivated the use of striatal and prefrontal regions of interest (ROIs) in the subsequent analysis of drug effects. The ROIs were defined from the regions significantly activated in the cue minus maintenance period group analysis, collapsed across drug and placebo. This contrast was used for two reasons: firstly, because it allowed us to investigate drug effects across the entire network involved in performing the task rather than just a subset of regions; secondly, because we observed no significant difference in activation in the contrast manipulation cue–simple retrieval cue averaged across the placebo and sulpiride sessions. We took the peak coordinates of activated clusters from the group analysis and constructed spheres with 5 mm radiuses centred on these coordinates. We used spheres, rather than the clusters themselves, because in some cases the clusters encompassed several activated regions which we wanted to examine separately. ROI construction and analysis was performed using the MarsBar ROI toolbox (Brett et al. 2002). We constructed seven separate ROIs: right and left putamen; right and left dorsolateral prefrontal cortex (DLPFC); right and left ventrolateral prefrontal cortex (VLPFC)/insula and anterior cingulate cortex (ACC). In order to reduce the chance of making a type 1 error we divided the p value required for rejection of the null hypothesis (0.05) by the number of ROIs (seven) to give a conservative p value of 0.007. For each ROI, a single BOLD measurement, reflecting the difference in activation between the manipulation cue and retrieval cue events, was obtained by running this contrast on each voxel within the ROI and then averaging across all the resulting t values.
In order to investigate whether sulpiride modulated task-related activation, the contrast images from the first-level contrast cue–maintenance were taken to a second-level analysis involving a paired-samples t test with drug (sulpiride or placebo) as the factor. To test our specific prediction that sulpiride would modulate activation during the manipulation of information in working memory, we computed a further contrast, manipulation cue–retrieval cue, at the subject-specific level, and the contrast images from this contrast were taken to a second-level analysis involving a paired-samples t test with drug as the factor.
In order to explore the effects of individual variation in drug uptake on task-related activation, the contrast images from the two first-level contrasts were taken to two further second-level simple regression analyses with sulpiride plasma levels as the single covariate of interest. The aim here was to test whether task-related activation was modulated by sulpiride plasma level and, more specifically, whether variation in plasma level predicted the difference between activation associated with the manipulation process and activation associated with the simple retrieval process.
Results
Drug effects on behavioural performance
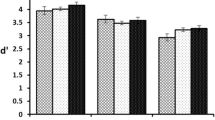
To analyse effects of sulpiride on accuracy on the working memory task, for each subject the proportion of correct responses in the retrieval and manipulation conditions was arcsine transformed and subjected to a repeated-measures ANOVA with drug (placebo and sulpiride) and condition (retrieval and manipulation) as the factors. There was a significant main effect of condition, F(1,16) = 7.7, p < 0.05, as subjects were more accurate on the retrieval trials than the manipulation trials. There was no main effect of drug, F(1,16) = 1.4, p = .25, but a significant interaction between drug and condition, F(1,16) = 5.9, p < 0.05. The nature of the interaction was qualified with post-hoc paired t tests comparing accuracy between the placebo and sulpiride conditions separately for the same and manipulation conditions. Accuracy was higher after sulpiride than after placebo in the manipulation condition, t(16) = −2.4, p < 0.05, but with no difference in the retrieval condition, t(16) = 0.06, p = 0.95. Accuracy data are displayed in Fig. 2.
To analyse effects of sulpiride on the speed of retrieval and manipulation, RTs to the cue were subjected to a repeated-measures ANOVA with drug (placebo and sulpiride) and condition (retrieval and manipulation) as the factors. There was a significant main effect of condition, F(1,16) = 117.6, p < 0.05, as mean RT was lower in the retrieval condition (924 ms) than in the manipulation condition (2,098 ms), but no main effect of drug, F(1,16) = 0.4, p = 0.5, and no interaction between drug and condition, F(1,16) = 0.5, p = 0.5.
fMRI data
Activation related to manipulation/simple retrieval processes
The results of the contrast cue–maintenance period, collapsing across the manipulation and simple retrieval conditions and across placebo and sulpiride sessions, revealed an extensive network of regions which showed significantly higher activity during the cue (Fig. 3). These included bilateral striatum (putamen), bilateral ventrolateral prefrontal cortex/insula, bilateral dorsolateral prefrontal cortex, anterior cingulate (ACC), bilateral inferior parietal lobe, midbrain and occipital cortex.
Cue-period activity independent of drug effects. Whole-brain statistical parametric maps (SPM) showing the results of the contrast cue minus maintenance period, performed at the random-effects level on the average of the first-level contrast images from the placebo and sulpiride conditions, superimposed on the Montreal Neurological Institute (MNI) template brain (p < 0.05 corrected for false discovery rate). Five axial slices are shown from different levels in the vertical plane. Z coordinates below slices indicate position of the slices in the vertical plane (far left slice is furthest dorsal, far right slice is furthest ventral). Task-related activation was found bilaterally in the striatum (putamen), DLPFC, VLPFC/insula, and ACC, as well as in the parietal cortex, occipital cortex and midbrain. The labels above the slices indicate which regions were selected for subsequent ROI analyses of drug effects
The contrast manipulation cue–simple retrieval cue, collapsed across the placebo and sulpiride sessions, did not reveal any significantly activated clusters.
Drug effects on task-related activation
In the paired-samples t test which examined the overall effect of sulpiride on task-related activation there was no significant interaction between drug and the contrast cue–maintenance in any of the ROIs tested, including L DLPFC: t(16) = 0.4, p = 0.35; L putamen: t(16) = 1.27, p = 0.11; R putamen: t(16) = 1.16, p = 0.1; ACC, t(16) = −0.7, p = 0.75; R VLPFC/insula, t(16) = −0.56, p = 0.71; L VLPFC/insula, t(16) = 0.48, p = 0.32. However the interaction approached significance in the R DLPFC, t(16) = 2.0, p = 0.03.
In the paired-samples t test which examined the overall effect of sulpiride on manipulation-specific activation, there was no significant interaction between drug and the contrast manipulation cue–retrieval cue in any of the ROIs tested, including L DLPFC: t(16) = 1.14, p = 0.14; R DLPFC t(16) = 0.15, p = 0.44; L putamen: t(16) = 0.66, p = 0.26; R putamen: t(16) = 1.0, p = 0.17. However, the interaction approached significance in the ACC, t(16) = 1.45, p = 0.08, R VLPFC/insula, t(16) = 1.99, p = 0.03 and L VLPFC/insula, t(16) = 2.87, p = 0.04
Plasma-dependent drug effects on task-related activation
The mean sulpiride plasma level across all subjects, averaged across the two samples (pre- and post-scanning), was 567 µg/l, and the SD was 295, with a range from 163 to 1,012 µg/l.
There was a significant negative correlation between plasma level and activation in the contrast manipulation cue–retrieval cue bilaterally in the putamen: left putamen (−24, 6, −6), t(16) = 3.53, p < 0.007; right putamen (24, 10, −8), t(16) = 2.83, p < 0.007. As Fig. 4 shows, the difference in activation between the manipulation and retrieval cues depended on sulpiride plasma level; subjects with lower sulpiride plasma levels showed greater activation when cued to manipulate the items in working memory than when cued to simply retrieve the items in working memory, while for subjects with higher plasma levels the pattern was reversed, with greater activation for the retrieval cue than for the manipulation cue. There was no significant correlation between plasma level and activation in this contrast in any of the other ROIs: L DLPFC t(16) = 0.42, p = 0.34; R DLPFC t(16) = 0.89, p = 0.19; ACC t(16) = 0.49, p = 0.31; R VLPFC/insula t(16) = −0.18, p = 0.57; L VLPFC/insula t(16) = 2.37, p = 0.01.
Scatterplot showing the negative correlation between sulpiride plasma levels and difference in activation between manipulation and simple retrieval trials in the left putamen. Lower sulpiride plasma levels are associated with positive t values (manipulation > retrieval), whilst higher sulpiride plasma levels are associated with negative t values (manipulation < retrieval). Individual subjects’ plasma values were calculated from the mean of the pre- and post- scanning values. Individual subjects’ t values reflect the effect size of the contrast manipulation cue–retrieval cue and were extracted by performing this contrast at the single-subject level on the 5 mm sphere ROI centred on the left putamen, MNI coordinates (−24, 6, −6)
There was no significant correlation between plasma level and activation in the contrast cue–maintenance in any of the ROIs: L DLPFC t(16) = 0.25, p = 0.40; R DLPFC t(16) = 0.97, p = 0.17; L putamen t(16) = 2.17, p = 0.02; R putamen t(16) = 1.09, p = 0.15; ACC t(16) = 0.00, p = 0.5; R VLPFC/insula, t(16) = 0.89, p = 0.19. However the correlation approached significance in the L VLPFC/insula, t(16) = 2.27, p = 0.02.
Drug effects on subjective alertness
In order to investigate the effects of sulpiride on subjective mood, three factors were calculated from the VAS scores—alertness, contentment and calmness (see Herbert et al. 1976, for the method of calculating the factors). Paired-samples t tests carried out on participants’ scores on these factors revealed no significant differences between the placebo and sulpiride conditions (p values all >0.3).
Correlations between activation and performance
In order to investigate whether the difference in activation between the manipulation and retrieval conditions correlated with manipulation accuracy or RT in the sulpiride condition, we performed two regression analyses on the first-level contrast images from the contrast manipulation cue–retrieval cue, one with manipulation accuracy as the regressor and one with manipulation (cue) RT as the regressor. There was no significant correlation with accuracy either in the left putamen, t(16) = 0.57, p = 0.29, or in the right putamen, t(16) = 0.41, p = 0.34. There was no significant correlation with RT either in the left putamen, t(16) = 0.89, p = 0.19, or in the right putamen, t(16) = 1.28, p = 0.1.
Correlation between plasma level and accuracy
In order to investigate whether sulpiride plasma levels predicted performance on the working memory task we performed bivariate correlations comparing RT and accuracy on the task with plasma levels. There was no significant correlation between plasma and accuracy either in the simple retrieval condition, r = 0.43, p = 0.08 or in the manipulation condition, r = −0.04, p = 0.88, and there was no significant correlation between plasma level and RT either in the simple retrieval condition, r = −0.02, p = 0.95, or in the manipulation condition, r = −0.01, p = 0.98.
Discussion
We examined the effects of the DA D2 receptor antagonist sulpiride on brain activation during performance of a verbal working memory task requiring different levels of manipulation. We observed no overall effect of sulpiride on task-related activation. However, as predicted, we observed a plasma-level-dependent effect of sulpiride on activation in the putamen associated with the manipulation of information in working memory. Lower sulpiride plasma levels were associated with greater activation in manipulation trials than in simple retrieval trials, whilst higher sulpiride plasma levels were associated with the opposite pattern-greater activation in simple retrieval trials than in manipulation trials. The results establish a key link between DA receptor antagonism and a striatally based system for manipulating information in working memory. They also highlight the importance of taking into account drug plasma levels in the analysis of pharmacological functional neuroimaging data.
The finding that sulpiride, a selective DA D2 receptor antagonist, modulated striatal activation during working memory manipulation is consistent with previous studies suggesting a link between DA D2 receptor signalling and the process of updating task-related working memory representations. For example, Mehta et al. (2004) found that sulpiride impaired task-set switching and attentional set shifting, tasks in which subjects are required to repeatedly update goal-related representations. Frank and O’Reilly (2006) found that cabergoline and haloperidol, both selective D2 agents, modulated the efficacy of working memory updating. Cools et al (2007) found that bromocriptine, a D2 receptor agonist, improved the flexible updating of working memory representations in trait impulsive subjects, and that these improvements were associated with modulations of striatal, but not prefrontal, activation. The present results support and extend these findings by showing that a selective DA D2/D3 receptor antagonist selectively modulated manipulation-related activation in a dose-dependent fashion.
The present data are also consistent with findings from PD, in which patients with a progressive loss of DA neurons from the striatum are impaired on tasks requiring the flexible updating of task-related representations (Owen et al. 1992, 1993; Cools et al. 2001a, b; Lewis et al. 2003). A recent PET study has provided strong evidence that these functional impairments are caused by striatal DA depletion (Sawamoto et al. 2008). Our results are consistent with this hypothesis, providing converging evidence for a link between striatal DA and flexible behaviour, and further suggest that the gradual loss of cognitive flexibility in PD may be due specifically to a loss of DA D2 receptors in the striatum.
It is well established that DA D1 signalling in the PFC plays an important role in maintaining stable working memory representations. However, several authors have hypothesised that DA plays a different role at the level of the striatum, facilitating the flexible updating of task-relevant information in working memory (Frank et al. 2001; Durstewitz and Seamans 2002; Bilder et al. 2004). The present data are consistent with this hypothesis. In simple retrieval trials, performance is dependent on retrieving the representation of the letter-string held in working memory during the delay period. In contrast, in manipulation trials subjects must rapidly reorder the letter-string according to the cue, a process requiring the selective updating of the contents of working memory. The modulation of manipulation-related activation by sulpiride suggests that this striatal ‘gating’ mechanism is specifically dependent on D2 receptor signalling. This is consistent with findings in rats that manipulation of tonic DA release selectively modulates PFC function through altered stimulation of D2 receptors (Goto and Grace 2005), and provides a potential mechanism of action for the therapeutic effects of antipsychotics such as sulpiride in schizophrenia.
A previous neuroimaging study of sulpiride found mixed results with respect to drug modulations of process-specific activity. Mehta et al. (2003) used PET to investigate the effects of sulpiride 400 mg on striatal rCBF during spatial working memory and planning. They found no effect of sulpiride on rCBF related specifically to working memory but some evidence that sulpiride attenuated rCBF in the caudate nucleus during planning and that the drug-related attenuation was associated with changes in performance. The lack of effect of sulpiride on working memory-related rCBF in that study may be due to differences in the sensitivity of different tasks to DA manipulations. However, it is also possible that the inability to separate out neural activation associated with specific cognitive processes using PET scanning caused more subtle modulations of process-specific activation to be masked.
It should be noted that the putamen region in which manipulation-specific activation correlated with sulpiride plasma levels did not show any selective task-related (i.e. independent of any drug effect) activation in the contrast of manipulation–retrieval cue periods. However, the putamen was activated more generally during the manipulation and retrieval cues when compared with the maintenance period. Thus, activation in the putamen appears to be sensitive to differences in task requirements in terms of the level of manipulation of information required, but only under conditions of partially blocked dopamine D2 receptor activity after sulpiride.
One possible explanation for this pattern of results is that putamen activation is, under normal conditions, relatively robust to changes in working memory manipulation demands, due to a high signal-to-noise ratio. However, a reduction in D2 receptor activity introduces noise into the system, rendering putamen activation more vulnerable to changes in working memory processing demands, and leading to differences in activation between the manipulation and retrieval cue conditions.
The present data highlight the importance of taking into account drug plasma levels in the analysis of pharmacological functional neuroimaging data, and in this respect complement previous findings of plasma-dependent effects of dopaminergic agents on task-related brain activation (Muller et al. 2005). Four hundred milligrams of sulpiride has been shown to lead to only 28% occupancy of DA D2 receptors in the striatum (Mehta et al. 2008) and 35–60% in extrastriatal regions (Takano et al. 2006); extrastriatal D2 receptor occupancy after sulpiride is dose- and plasma-level-dependent. Thus, variation in plasma levels will likely lead to difficulties detecting small overall differences in activation between conditions. Furthermore, plasma levels may vary according to many incidental and uncontrollable factors, including: individual differences in drug pharmacokinetics (rate of absorption, time of peak plasma concentration); food intake prior to testing (despite attempts to control for this factor, subjects may not follow instructions); variation in the time between dosing and first blood sample; and variation in scanning time (despite attempts to keep these final two factors consistent, unavoidable events can lead to small differences between subjects). Despite the lack of an overall drug effect, our data show that careful measurement of plasma levels can strengthen a study by providing a clear dose–response relationship, which, in human pharmacological studies is often difficult to achieve due to ethical and financial constraints.
It should be noted that one limitation in interpretation of the present findings is that sulpiride, in common with virtually all ‘selective‘ D2 receptor antagonists and agonists, exhibits affinity for the D3 as well as the D2 receptor (Levant 1997). Although DA D3 receptors appear to be preferentially located to the ventral rather than the dorsal striatum, there is evidence of some expression of D3 receptors in the putamen (Schwartz et al 2000) and so it cannot be excluded that some of the effects of sulpiride described here are due its antagonism of that receptor.
Alongside the neural effects of sulpiride, we observed a drug-induced improvement in accuracy in the manipulation condition but not in the retrieval condition, suggesting a selective effect of drug on the process of manipulation. It should be noted that accuracy in the retrieval condition was already high after placebo, and may therefore have been insensitive to pharmacological manipulation. It is nevertheless interesting that sulpiride led to an improvement rather than a reduction in accuracy in the manipulation condition.
This behavioural improvement is not without precedent. In humans, studies involving DA D2 antagonists such as sulpiride and haloperidol have shown that these drugs can lead to enhanced performance on certain working memory and executive tasks, especially those with a strategic (Mehta et al. 2003; Mehta et al. 2004) or incentive learning (Frank and O’Reilly 2006) component. These results are reminiscent of findings that the dopamine precursor L-dopa and dopamine agonists such as bromocriptine can paradoxically lead to impairments on certain tasks such as reversal learning while leading to improvements on other tasks such as spatial memory in healthy volunteers (Mehta et al. 2001) and task-switching in PD (Cools et al. 2001a, b, 2003). These data have been interpreted as reflecting an ‘overdosing’ of the DA system, whereby an increase in DA can lead to impairments on tasks that require lower levels of DA for optimal performance whilst leading to improvements on tasks that require higher levels of DA for optimal performance (Cools and Robbins 2004). In a similar fashion, the present increase in accuracy in the manipulation condition may be due to the fact that sulpiride reduces DA signalling to the optimal level required for performance of the present manipulation task.
An alternative explanation for the improvement in behavioural performance after sulpiride is that it is actually due to a drug-induced increase in extracellular DA. Animal studies have shown that relatively low single doses of DA antagonists such as sulpiride and haloperidol increase levels of striatal DA (Imperato and Di Chiara 1985; Moghaddam and Bunney 1990; Kawagoe et al. 1992) by inhibiting the presynaptic activity of DA autoreceptors (Garris et al. 2003). This increased DA will hit D1 receptors and thus behavioural improvements after sulpiride may in fact be attributable to the increase in extracellular striatal DA induced by downregulation of presynaptic activity. Indeed, Frank and O’Reilly (2006) interpret their finding that haloperidol enhanced incentive learning in this context, and further hypothesise that previous findings of sulpiride-induced impairments on attentional switching may also be due to an increase in DA causing excessive attention to be paid to the previously irrelevant dimension. This interpretation is also consistent with findings that sulpiride increases connectivity between the ventral midbrain and striatum and between the striatum and thalamus (Honey et al. 2003). Thus, the present sulpiride-induced improvement in accuracy during the manipulation of information in working memory may be due to an increase in extracellular striatal DA leading to enhanced attention to task-relevant information.
A final possible explanation for the improvement in accuracy is that striatal activation is actually detrimental to the process of manipulating information in working memory. Previous studies have associated striatal activation with the learning of simple stimulus–response mappings (Graybiel 2008). If this is the case, then simple retrieval and more complex manipulation processes may act mutually antagonistically, such that improvement in one process leads to impairment in the other. In the present example, sulpiride may reduce the ability to respond via a simple S-R strategy and consequently improve the ability to adopt a more flexible approach to the working memory task. This explanation, whilst speculative, has the advantage of linking the drug effects on striatal activation with the observed behavioural effects.
In summary, we observed a dose-dependent effect of sulpiride on manipulation-related activation bilaterally in the striatum in the context of an improvement in accuracy on manipulation trials. The results are consistent with the hypothesis that DA D2 receptor signalling in the striatum performs a gating function, enabling or preventing the updating of currently relevant working memory representations.
References
Bilder RM, Volavka J, Lachman HM, Grace AA (2004) The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology 29(11):1943–1961
Bond A, Lader M (1974) The use of visual analogue scales in rating subjective feelings. Br J Med Psychol 47:211–218
Braver TS, Barch DM, Cohen JD (1999) Cognition and control in schizophrenia: a computational model of dopamine and prefrontal function. Biol Psychiatry 46(3):312–328
Brett M, Anton JL, Valabregue V, Poline JB (2002) Region of interest analysis using an SPM toolbox. Presented at the 8th International Conference on Functional Mapping of the Human Brain
Brozoski TJ, Brown RM, Rosvold HE, Goldman PS (1979) Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science 205(4409):929–932
Bublak P, Müller U, Grön G, Reuter M, von Cramon DY (2002) Difficult manipulation of working memory information is impaired in Parkinson’s disease and related to working memory capacity. Neuropsychology 16:577–590
Cools R, Robbins TW (2004) Chemistry of the adaptive mind. Philos Transact A Math Phys Eng Sci 362(1825):2871–2888
Cools R, Barker RA, Sahakian BJ, Robbins TW (2001a) Enhanced or impaired cognitive function in Parkinson’s disease as a function of dopaminergic medication and task demands. Cereb Cortex 11(12):1136–1143
Cools R, Barker RA, Sahakian BJ, Robbins TW (2001b) Mechanisms of cognitive set flexibility in Parkinson’s disease. Brain 124(Pt 12):2503–2512
Cools R, Barker RA, Sahakian BJ, Robbins TW (2003) L-Dopa medication remediates cognitive inflexibility, but increases impulsivity in patients with Parkinson’s disease. Neuropsychologia 41(11):1431–1441
Cools R, Sheridan M, Jacobs E, D’Esposito M (2007) Impulsive personality predicts dopamine-dependent changes in frontostriatal activity during component processes of working memory. J Neurosci 27(20):5506–5514
Crofts HS, Dalley JW, Collins P, Van Denderen JC, Everitt BJ, Robbins TW et al (2001) Differential effects of 6-OHDA lesions of the frontal cortex and caudate nucleus on the ability to acquire an attentional set. Cereb Cortex 11(11):1015–1026
Cusack R, Brett M, Osswald K (2003) An evaluation of the use of magnetic field maps to undistort echo-planar images. Neuroimage 18(1):127–142
Dodds CM, Müller U, Clark L, van Loon A, Cools R, Robbins TW (2008) Methylphenidate has differential effects on blood oxygenation level-dependent signal related to cognitive subprocesses of reversal learning. J Neurosci 28(23):5976–5982
Durstewitz D, Seamans JK (2002) The computational role of dopamine D1 receptors in working memory. Neural Netw 15(4–6):561–572
Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MT (2006) Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology 31(2):297–309
Frank MJ, O’Reilly RC (2006) A mechanistic account of striatal dopamine function in human cognition: psychopharmacological studies with cabergoline and haloperidol. Behav Neurosci 120(3):497–517
Frank MJ, Loughry B, O’Reilly RC (2001) Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cogn Affect Behav Neurosci 1(2):137–160
Friston KJ, Holmes AP, Worsley KJ, Poline J-B, Frith CD, Frackowiak RSJ (1995) Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2(4):189–210
Garris PA, Budygin EA, Phillips PE, Venton BJ, Robinson DL, Bergstrom BP et al (2003) A role for presynaptic mechanisms in the actions of nomifensine and haloperidol. Neuroscience 118(3):819–829
Goto Y, Grace AA (2005) Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci 8(6):805–812
Graybiel AM (2008) Habits, rituals, and the evaluative brain. Annu Rev Neurosci 31:359–387
Gschwend MH, Arnold P, Ring J, Martin W (2006) Selective and sensitive determination of amisulpride in human plasma by liquid chromatography-tandem mass spectrometry with positive electrospray ionisation and multiple reaction monitoring. J Chromatogr B Analyt Technol Biomed Life Sci 831(1–2):132–139
Herbert M, Johns MW, Dore C (1976) Factor analysis of analogue scales measuring subjective feelings before and after sleep. Br J Med Psychol 49(4):373–379
Honey GD, Suckling J, Zelaya F, Long C, Routledge C, Jackson S et al (2003) Dopaminergic drug effects on physiological connectivity in a human cortico-striato-thalamic system. Brain 126(Pt 8):1767–1781
Imperato A, Di Chiara G (1985) Dopamine release and metabolism in awake rats after systemic neuroleptics as studied by trans-striatal dialysis. J Neurosci 5(2):297–306
Kawagoe KT, Garris PA, Wiedemann DJ, Wightman RM (1992) Regulation of transient dopamine concentration gradients in the microenvironment surrounding nerve terminals in the rat striatum. Neuroscience 51(1):55–64
Levant B (1997) The D3 dopamine receptor: neurobiology and potential clinical relevance. Pharmacol Rev 49:231–252
Lewis SJ, Cools R, Robbins TW, Dove A, Barker RA, Owen AM (2003) Using executive heterogeneity to explore the nature of working memory deficits in Parkinson’s disease. Neuropsychologia 41(6):645–654
Lewis SJ, Dove A, Robbins TW, Barker RA, Owen AM (2004) Striatal contributions to working memory: a functional magnetic resonance imaging study in humans. Eur J Neurosci 19(3):755–760
Mehta MA, Sahakian BJ, McKenna PJ, Robbins TW (1999) Systemic sulpiride in young adult volunteers simulates the profile of cognitive deficits in Parkinson’s disease. Psychopharmacology (Berl) 146(2):162–174
Mehta MA, Swainson R, Ogilvie AD, Sahakian J, Robbins TW (2001) Improved short-term spatial memory but impaired reversal learning following the dopamine D(2) agonist bromocriptine in human volunteers. Psychopharmacology (Berl) 159(1):10–20
Mehta MA, McGowan SW, Lawrence AD, Aitken MR, Montgomery AJ, Grasby PM (2003) Systemic sulpiride modulates striatal blood flow: relationships to spatial working memory and planning. Neuroimage 20(4):1982–1994
Mehta MA, Manes FF, Magnolfi G, Sahakian BJ, Robbins TW (2004) Impaired set-shifting and dissociable effects on tests of spatial working memory following the dopamine D2 receptor antagonist sulpiride in human volunteers. Psychopharmacology (Berl) 176(3–4):331–342
Mehta MA, Montgomery AJ, Kitamura Y, Grasby PM (2008) Dopamine D2 receptor occupancy levels of acute sulpiride challenges that produce working memory and learning impairments in healthy volunteers. Psychopharmacology (Berl) 196(1):157–165
Moghaddam B, Bunney BS (1990) Utilization of microdialysis for assessing the release of mesotelencephalic dopamine following clozapine and other antipsychotic drugs. Prog Neuropsychopharmacol Biol Psychiatry 14(Suppl):S51–S57
Müller U, Suckling J, Zelaya F, Honey G, Faessel H, Williams SC et al (2005) Plasma level-dependent effects of methylphenidate on task-related functional magnetic resonance imaging signal changes. Psychopharmacology 180(4):624–633
Owen AM (2004) Cognitive dysfunction in Parkinson’s disease: the role of frontostriatal circuitry. Neuroscientist 10(6):525–537
Owen AM, James M, Leigh PN, Summers BA, Marsden CD, Quinn NP et al (1992) Fronto-striatal cognitive deficits at different stages of Parkinson’s disease. Brain 115(Pt 6):1727–1751
Owen AM, Beksinska M, James M, Leigh PN, Summers BA, Marsden CD et al (1993) Visuospatial memory deficits at different stages of Parkinson’s disease. Neuropsychologia 31(7):627–644
Sawamoto N, Piccini P, Hotton G, Pavese N, Thielemans K, Brooks DJ (2008) Cognitive deficits and striato-frontal dopamine release in Parkinson’s disease. Brain 131(Pt 5):1294–1302
Schwartz J-C, Dias J, Pilon C, Sokoloff P (2000) Possible implications of the dopamine D3 receptor in schizophrenia and in anti-psychotic drug actions. Brain Res Rev 31:277–287
Smith SM (2002) Fast robust automated brain extraction. Hum Brain Mapp 17(3):143–155
Sugnaux FR, Benakis A, Fonzo D, Di Carol R (1983) Dose-dependent pharmacokinetics of sulpiride and sulpiride-induced prolactin secretion in man. Eur J Drug Metab Pharmacokinet 8:189–200
Takano A, Suhara T, Yasuno F, Suzuki K, Takahashi H, Morimoto T, Lee YJ, Kusuhara H, Sugiyama Y, Okubo Y (2006) The antipsychotic sultopride is overdosed—a PET study of drug-induced receptor occupancy in comparison with sulpiride. Int J Neuropsychopharmacol 9(5):539–545
Wagstaff AJ, Fitton A, Benfield P (1994) Supiride: a review if its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in schizophrenia. CNS Drugs 2:313–333
Acknowledgements
We would like to thank all participants, nurses and administrative staff at the Wellcome Trust Clinical Research Facility and Vicky Lupton and her team at the Wolfson Brain Imaging Centre. This research was funded by a Programme Grant (no. 076274/4/Z/04/Z) awarded by the Wellcome Trust to TWR, BJ Everitt, AC Roberts and BJ Sahakian and completed within the University of Cambridge Behavioural and Clinical Neuroscience Institute (BCNI) funded by a joint award from the Medical Research Council and the Wellcome Trust. UM was supported by a Feodoy-Lynen-Fellowship awarded by the Alexander von Humboldt Foundation and the Isaac Newton Trust, Cambridge.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Dodds, C.M., Clark, L., Dove, A. et al. The dopamine D2 receptor antagonist sulpiride modulates striatal BOLD signal during the manipulation of information in working memory. Psychopharmacology 207, 35–45 (2009). https://doi.org/10.1007/s00213-009-1634-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-009-1634-0