Abstract
Rationale
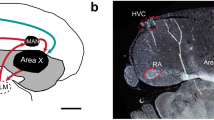
CB1 cannabinoid receptors are distinctly expressed at high density within several regions of zebra finch telencephalon, including those known to be involved in song learning (lMAN and Area X) and production (HVC and RA) because (1) exposure to cannabinoid agonists during developmental periods of auditory and sensory-motor song learning alters song patterns produced later in adulthood and (2) densities of song region expression of CB1 waxes and wanes during song learning. It is becoming clear that CB1-receptor-mediated signaling is important to normal processes of vocal development.
Materials and methods
To better understand the mechanisms involved in cannabinoid modulation of vocal behavior, we have investigated the dose–response relationship between systemic cannabinoid exposure and changes in neuronal activity (as indicated by expression of the transcription factor, c-Fos) within telencephalic brain regions, with established involvement in song learning and/or control.
Results
In adults, we have found that low doses (0.1 mg/kg) of the cannabinoid agonist WIN-55212-2 decrease neuronal activity (as indicated by densities of c-fos-expressing nuclei) within vocal motor regions of caudal telencephalon (HVC and RA) while higher doses (3 mg/kg) stimulate activity. Both effects were reversed by pretreatment with the CB1-selective antagonist rimonabant. Interestingly, no effects of cannabinoid treatment were observed within the rostral song regions lMAN and Area X, despite distinct and dense CB1 receptor expression within these areas.
Conclusions
Overall, our results demonstrate that, depending on dosage, CB1 agonism can both inhibit and stimulate neuronal activity within brain regions controlling adult vocal motor output, implicating involvement of multiple CB1-sensitive neuronal circuits.






Similar content being viewed by others
Abbreviations
- lMAN:
-
lateral magnocellular nucleus of the anterior nidopallium
- Area X:
-
area X within songbird medial striatum
- RA:
-
robust nucleus of the arcopallium
- Uva:
-
nucleus uvaformis
- DLM:
-
dorsal lateral nucleus of the medial thalamus
- HVC:
-
used as a proper name (per Reiner et al. 2004a, b) to indicate a prominent vocal motor nucleus of zebra finch telencephalon
References
Bolhuis JJ, Hetebrij E, Den Boer-Visser AM, De Groot JH, Zijlstra GGO (2001) Localized immediate early gene expression related to the strength of song learning in socially reared zebra finches. Eur J Neurosci 13:2165–2170
Bottjer SW, Johnson F (1997) Circuits, hormones, and learning: vocal behavior in songbirds. J Neurobiol 33:602–618
Bottjer SW, Miesner EA, Arnold AP (1984) Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science 224:901–903
Chaperon F, Thiebot MH (1999) Behavioral effects of cannabinoid agents in animals. Crit Rev Neurobiol 13:243–281
Doupe AJ, Kuhl PK (1999) Bird song and human speech: common themes and mechanisms. Annu Rev Neurosci 22:567–631
Elphick MR, Egertova M (2001) The neurobiology and evolution of cannabinoid signalling. Philos Trans R Soc Lond B Biol Sci 356:381–408
Fowler CJ (2007) The pharmacology of the cannabinoid system—a question of efficacy and selectivity. Mol Neurobiol 36:15–25
Mackie K, Hille B (1992) Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proc Natl Acad Sci USA 89:3825–3829
Mackie K, Lai Y, Westenbroek R, Mitchell R (1995) Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J Neurosci 15:6552–6561
Mailleux P, Verslype M, Preud’homme X, Vanderhaeghen JJ (1994) Activation of multiple transcription factor genes by tetrahydrocannabinol in rat forebrain. Neuroreport 5:1265–1268
McGregor IS, Arnold JC, Weber MF, Topple AN, Hunt GE (1998) A comparison of delta 9-THC and anandamide induced c-fos expression in the rat forebrain. Brain Res 802:19–26
Nottebohm F, Stokes TM, Leonard CM (1976) Central control of song in the canary, Serinus canarius. J Comp Neurol 165:457–486
Patel S, Hillard CJ (2003) Cannabinoid-induced Fos expression within A10 dopaminergic neurons. Brain Res 963:15
Patel NA, Moldow RL, Patel JA, Wu G, Chang SL (1998) Arachidonylethanolamide (AEA) activation of FOS proto-oncogene protein immunoreactivity in the rat brain. Brain Res 797:225–233
Pertwee RG (1997) Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther 74:129–180
Pertwee RG (2005) Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci 76:1307–1324
Peterson RS, Yarram L, Schlinger BA, Saldanha CJ (2005) Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proc Biol Sci 272:2089–2096
Reiner A, Laverghetta AV, Meade CA, Cuthbertson SL, Bottjer SW (2004a) An immunohistochemical and pathway tracing study of the striatopallidal organization of area X in the male zebra finch. J Comp Neurol 469:239–261
Reiner AP, Perkel DJ, Bruce L, Butler AB, Csillag A, Kunzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Güntürkün O, Lee DW, Mello CV, Powers A, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis ED (2004b) Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol 473:377–414
Reiner A, Yamamoto K, Karten HJ (2005) Organization and evolution of the avian forebrain. Anat Rec A Discov Mol Cell Evol Biol 287A:1080–1102
Scharff C, Nottebohm F (1991) A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. J Neurosci 11(9):2896–2913
Soderstrom K, Johnson F (2001) The zebra finch CB1 cannabinoid receptor: pharmacology and in vivo and in vitro effects of activation. J Pharmacol Exp Ther 297:189–197
Soderstrom K, Johnson F (2003) Cannabinoid exposure alters learning of zebra finch vocal patterns. Brain Res Dev Brain Res 142:215–217
Soderstrom K, Tian Q (2004) Distinct periods of cannabinoid sensitivity during zebra finch vocal development. Dev Brain Res 153:225–232
Soderstrom K, Tian Q (2006) Developmental pattern of CB1 cannabinoid receptor immunoreactivity in brain regions important to zebra finch (Taeniopygia guttata) song learning and control. J Comp Neurol 496:739–758
Soderstrom K, Leid M, Moore FL, Murray TF (2000) Behavioral, pharmacological and molecular characterization of an amphibian cannabinoid receptor. J Neurochem 75:413–423
Soderstrom K, Tian Q, Valenti M, Di Marzo V (2004) Endocannabinoids link feeding state and auditory perception-related gene expression. J Neurosci 24:10013–10021
Soderstrom K, Qin W, Leggett MH (2007) A minimally invasive procedure for sexing young zebra finches. J Neurosci Methods 164:116–119
Spear L (2000) Modeling adolescent development and alcohol use in animals. Alcohol Res Health 24:115–123
Troyer TW, Bottjer SW (2001) Bird song: models and mechanisms. Curr Opin Neurobiol 11(6):721–726
Whitney O, Soderstrom K, Johnson F (2003) CB1 cannabinoid receptor activation inhibits a neural correlate of song recognition in an auditory/perceptual region of the zebra finch telencephalon. J Neurobiol 56:266–274
Wilson RI, Nicoll RA (2002) Endocannabinoid signaling in the brain. Science 296:678–682
Acknowledgment
We are grateful to Bin Luo who managed the breeding aviary and assisted in these experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by NIDA grants R01DA020109 and R21DA14693.
Rights and permissions
About this article
Cite this article
Soderstrom, K., Tian, Q. CB1 cannabinoid receptor activation dose dependently modulates neuronal activity within caudal but not rostral song control regions of adult zebra finch telencephalon. Psychopharmacology 199, 265–273 (2008). https://doi.org/10.1007/s00213-008-1190-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-008-1190-z