Abstract
Rationale
The endocannabinoid system plays a crucial role in the control of emotionality and recent clinical findings have shown that heavy prenatal exposure to cannabis is significantly associated with self-reported anxiety symptoms in exposed children. However, the long-term neurobehavioral consequences of in utero exposure to low–moderate doses of cannabinoid compounds have never been investigated.
Objective
The objective of this study was to investigate whether perinatal exposure to moderate doses of the active constituent of cannabis, the CB1 cannabinoid receptor agonist delta-9-tetrahydrocannabinol (THC), influences the emotional reactivity of rat offspring.
Methods
Primiparous Wistar rats were treated during pregnancy and lactation with doses of THC equivalent to the current estimates of moderate cannabis consumption in humans (2.5–5 mg kg−1, per os, from gestational day 15 to postnatal day 9). The emotional reactivity of infant, adolescent, and adult offspring was investigated using the isolation-induced ultrasonic vocalization, social interaction, and elevated plus-maze tests, respectively.
Results
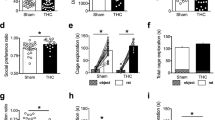
Perinatal THC treatment did not affect parameters of reproduction; however, at the dose of 5 mg kg−1, it increased the number of ultrasounds emitted by rat pups removed from the nest, inhibited social interaction and play behavior in the adolescent offspring, and induced an anxiogenic-like profile in the adult offspring tested in the elevated plus-maze test.
Conclusion
These results suggest that the endocannabinoid system is involved in the control of emotionality since early developmental stages. Thus, even moderate doses of cannabinoid compounds, when administered during the perinatal period, can have profound consequences for brain maturation, leading to long-lasting neurodevelopmental alterations.



Similar content being viewed by others
References
Adamec R, Kent P, Anisman H, Shallow T, Merali Z (1998) Neural plasticity, neuropeptides and anxiety in animals—implications for understanding and treating affective disorder following traumatic stress in humans. Neurosci Biobehav Rev 23:301–318
Ameri A (1999) The effects of cannabinoids on the brain. Prog Neurobiol 58:315–348
Antonelli T, Tomasini MC, Tattoli M, Cassano T, Tanganelli S, Finetti S, Mazzoni E, Trabace L, Steardo L, Cuomo V, Ferraro L (2005) Prenatal exposure to the CB1 receptor agonist WIN 55,212-2 causes learning disruption associated with impaired cortical NMDA receptor function and emotional reactivity changes in rat offspring. Cereb Cortex 15:2013–2020
Berrendero F, Sepe N, Ramos JA, Di Marzo V, Fernandez-Ruiz JJ (1999) Analysis of cannabinoid receptor binding and mRNA expression and endogenous cannabinoid contents in the developing rat brain during late gestation and early postnatal period. Synapse 33:181–191
Bortolato M, Campolongo P, Mangieri RA, Scattoni ML, Frau R, Trezza V, La Rana G, Russo R, Calignano A, Gessa GL, Cuomo V, Piomelli D (2006) Anxiolytic-like properties of the anandamide transport inhibitor AM404. Neuropsychopharmacology 31:2652–2659
Branchi I, Santucci D, Alleva E (2006) Analysis of ultrasonic vocalizations emitted by infant rodents. In: Costa LG, Hodgson E, Lawrence DA, Reed DJ (eds) Current protocols in toxicology. Wiley, Hoboken
Bromley BL, Rabii J, Gordon JH, Zimmerman E (1978) Delta-9-tetrahydrocannabinol inhibition of suckling-induced prolactin release in the lactating rat. Endocr Res Commun 5:271–278
Buckley NE, Hansson S, Harta G, Mezey E (1998) Expression of the CB1 and CB2 receptor messenger RNAs during embryonic development in the rat. Neuroscience 82:1131–1149
Calamandrei G, Venerosi A, Branchi I, Valanzano A, Puopolo M, Alleva E (1999) Neurobehavioral effects of prenatal lamivudine (3TC) exposure in preweaning mice. Neurotoxicol Teratol 21:365–373
Carobrez AP, Teixeira KV, Graeff FG (2001) Modulation of defensive behavior by periaqueductal gray NMDA/glycine-B receptor. Neurosci Biobehav Rev 25:697–709
Costa LG, Steardo L, Cuomo V (2004) Structural effects and neurofunctional sequelae of developmental exposure to psychotherapeutic drugs: experimental and clinical aspects. Pharmacol Rev 56:103–147
Cuomo V, De Salvia MA, Maselli MA, Santo L, Cagiano R (1987) Ultrasonic calling in rodents: a new experimental approach in behavioural toxicology. Neurotoxicol Teratol 9:157–160
D’Amato FR, Scalera E, Sarli C, Moles A (2005) Pups call, mothers rush: does maternal responsiveness affect the amount of ultrasonic vocalizations in mouse pups? Behav Genet 35:103–112
Davies SN, Pertwee RG, Riedel G (2002) Functions of cannabinoid receptors in the hippocampus. Neuropharmacology 42:993–1007
Elsner J, Suter D, Alder S (1990) Microanalysis of ultrasound vocalizations of young rats: assessment of the behavioral teratogenicity of methylmercury. Neurotoxicol Teratol 12:7–14
Farrell WJ, Alberts JR (2002) Stimulus control of maternal responsiveness to Norway rat (Rattus norvegicus) pup ultrasonic vocalizations. J Comp Psychol 116:297–307
Fernandez-Ruiz J, Berrendero F, Hernandez ML, Ramos JA (2000) The endogenous cannabinoid system and brain development. Trends Neurosci 23:14–20
Fernandez-Ruiz J, Gomez M, Hernandez M, de Miguel R, Ramos JA (2004) Cannabinoids and gene expression during brain development. Neurotox Res 6:389–401
File SE, Hyde JR (1978) Can social interaction be used to measure anxiety? Br J Pharmacol 62:19–24
Fride E, Mechoulam R (1996) Developmental aspects of anandamide: ontogeny of response and prenatal exposure. Psychoneuroendocrinology 21:157–172
Fried PA (1980) Marihuana use by pregnant women: neurobehavioral effects in neonates. Drug Alcohol Depend 6:415–424
Fried PA (1989a) Cigarettes and marijuana: are there measurable long-term neurobehavioral teratogenic effects? Neurotoxicology 10:577–583
Fried PA (1989b) Postnatal consequences of maternal marijuana use in humans. Ann N Y Acad Sci 562:123–132
Fried PA (2002a) Adolescents prenatally exposed to marijuana: examination of facets of complex behaviors and comparisons with the influence of in utero cigarettes. J Clin Pharmacol 42:97S–102S
Fried PA (2002b) Conceptual issues in behavioral teratology and their application in determining long-term sequelae of prenatal marihuana exposure. J Child Psychol Psychiatry 43:81–102
Fried PA, Smith AM (2001) A literature review of the consequences of prenatal marihuana exposure. An emerging theme of a deficiency in aspects of executive function. Neurotoxicol Teratol 23:1–11
Fried PA, Watkinson B, Gray R (2003) Differential effects on cognitive functioning in 13- to 16-year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol 25:427–436
Goldschmidt L, Day NL, Richardson GA (2000) Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicol Teratol 22:325–336
Goldschmidt L, Richardson GA, Cornelius MD, Day NL (2004) Prenatal marijuana and alcohol exposure and academic achievement at age 10. Neurotoxicol Teratol 26:521–532
Golub MS, Sassenrath EN, Chapman LF (1981) Mother–infant interaction in rhesus monkeys treated clinically with delta-9-tetrahydrocannabinol. Child Dev 52:389–392
Gray KA, Day NL, Leech S, Richardson GA (2005) Prenatal marijuana exposure: effect on child depressive symptoms at ten years of age. Neurotoxicol Teratol 27:439–448
Handley SL, Mithani S (1984) Effects of alpha-adrenoceptor agonists and antagonists in a maze-exploration model of ‘fear’-motivated behaviour. Naunyn Schmiedebergs Arch Pharmacol 327:1–5
Harkany T, Guzmán M, Galve-Roperh I, Berghuis P, Devi LA, Mackie K (2007) The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol Sci 28:83–92
Hermann H, Marsicano G, Lutz B (2002) Coexpression of the cannabinoid receptor type 1 with dopamine and serotonin receptors in distinct neuronal subpopulations of the adult mouse forebrain. Neuroscience 109:451–460
Hutchings DE, Martin BR, Gamagaris Z, Miller N, Fico T (1989) Plasma concentrations of delta-9-tetrahydrocannabinol in dams and fetuses following acute or multiple prenatal dosing in rats. Life Sci 44:697–701
Insel TR (2003) Is social attachment an addictive disorder? Physiol Behav 79:351–357
Insel TR, Hill JL, Mayor RB (1986) Rat pup ultrasonic isolation calls: possible mediation by the benzodiazepine receptor complex. Pharmacol Biochem Behav 24:1263–1267
Jakubovic A, Hattori T, McGeer PL (1977) Radioactivity in suckled rats after giving 14C-tetrahydrocannabinol to the mother. Eur J Pharmacol 22:221–223
Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, Freund TF (2001) Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci 21:9506–9518
Lamprea MR, Cardenas FP, Silveira R, Morato S, Walsh TJ (2000) Dissociation of memory and anxiety in a repeated elevated plus maze paradigm: forebrain cholinergic mechanisms. Behav Brain Res 117:97–105
Laviola G, Renna G, Bignami G, Cuomo V (1988) Ontogenetic and pharmacological dissociation of various components of locomotor activity and habituation in the rat. Int J Dev Neurosci 6:431–438
Leech S, Larkby CA, Day R, Day NL (2006) Predictors and correlates of high levels of depression and anxiety symptoms among children at age 10. J Am Acad Child Adolesc Psychiatry 45:223–230
Lester BM (1987) Developmental outcome prediction from acoustic cry analysis in term and preterm infants. Pediatrics 80:529–534
MacLean P (1990) The triune brain in evolution: role in paleocerebral functions. Plenum, New York
Maier SE, Miller JA, Blackwell JM, West JR (1999) Fetal alcohol exposure and temporal vulnerability: regional differences in cell loss as a function of the timing of binge-like alcohol exposure during brain development. Alcohol Clin Exper Res 23:726–734
Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O (2002) Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology (Berl) 159:379–387
McGregor IS, Dastur FN, McLellan RA, Brown RE (1996) Cannabinoid modulation of rat pup ultrasonic vocalizations. Eur J Pharmacol 313:43–49
Mereu G, Fa M, Ferraro L, Cagiano R, Antonelli T, Tattoli M, Ghiglieri V, Tanganelli S, Gessa GL, Cuomo V (2003) Prenatal exposure to a cannabinoid agonist produces memory deficits linked to dysfunction in hippocampal long-term potentiation and glutamate release. Proc Natl Acad Sci U S A 100:4915–4920
Michelsson K, Sirviö P, Wasz-Höckert O (1977) Sound spectrographic cry analysis of infants with bacterial meningitis. Dev Med Child Neurol 3:309–315
Millan MJ (2003) The neurobiology and control of anxious states. Prog Neurobiol 70:83–244
Molina-Holgado F, Molina-Holgado E, Leret ML, Gonzalez MI, Reader TA (1993) Distribution of indoleamines and [3H]paroxetine binding in rat brain regions following acute or perinatal delta 9-tetrahydrocannabinol treatments. Neurochem Res 18:1183–1191
Molina-Holgado F, Amaro A, Gonzalez MI, Alvarez FJ, Leret ML (1996) Effect of maternal delta 9-tetrahydrocannabinol on developing serotonergic system. Eur J Pharmacol 316:39–42
Moreno M, Trigo JM, Escuredo L, Rodriguez de Fonseca F, Navarro M (2003) Perinatal exposure to delta 9-tetrahydrocannabinol increases presynaptic dopamine D2 receptor sensitivity: a behavioral study in rats. Pharmacol Biochem Behav 75:565–575
Navarro M, Rubio P, de Fonseca FR (1995) Behavioural consequences of maternal exposure to natural cannabinoids in rats. Psychopharmacology (Berl) 122:1–14
Noirot E (1972) Ultrasounds and maternal behavior in small rodents. Dev Psychobiol 5:371–387
Onaivi ES, Green MR, Martin BR (1990) Pharmacological characterization of cannabinoids in the elevated plus maze. J Pharmacol Exp Ther 253:1002–1009
O’Shea M, McGregor IS, Mallet PE (2006) Repeated cannabinoid exposure during perinatal, adolescent or early adult ages produces similar long-lasting deficits in object recognition and reduced social interaction in rats. J Psychopharmacol 20(5):611–621
Paria BC, Dey SK (2000) Ligand-receptor signaling with endocannabinoids in preimplantation embryo development and implantation. Chem Phys Lipids 108:211–220
Pellow S, File SE (1986) Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav 24:525–529
Pellow S, Chopin P, File SE, Briley M (1985) Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14:149–167
Rasmussen DD, Mitton DR, Green J, Puchalski S (2001) Chronic daily ethanol and withdrawal: 2. Behavioral changes during prolonged abstinence. Alcohol Clin Exp Res 25:999–1005
Rodriguez de Fonseca F, Ramos JA, Bonnin A, Fernandez-Ruiz JJ (1993) Presence of cannabinoid binding sites in the brain from early postnatal ages. Neuroreport 4:135–138
Rodriguez de Fonseca F, Rubio P, Menzaghi F, Merlo-Pich E, Rivier J, Koob GF, Navarro M (1996) Corticotropin-releasing factor (CRF) antagonist [D-Phe12,Nle21,38,C alpha MeLeu37]CRF attenuates the acute actions of the highly potent cannabinoid receptor agonist HU-210 on defensive-withdrawal behavior in rats. J Pharmacol Exp Ther 276:56–64
Rodriguez de Fonseca F, Carrera MR, Navarro M, Koob GF, Weiss F (1997) Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science 276:2050–2054
Rubio P, Rodriguez de Fonseca F, Munoz RM, Ariznavarreta C, Martin-Calderon JL, Navarro M (1995) Long-term behavioral effects of perinatal exposure to delta 9-tetrahydrocannabinol in rats: possible role of pituitary-adrenal axis. Life Sci 56:2169–2176
Schlicker E, Kathmann M (2001) Modulation of transmitter release via presynaptic cannabinoid receptors. Trends Pharmacol Sci 22:565–572
Sloan LB, Gay JW, Snyder SW, Bales WR (1992) Substance abuse during pregnancy in a rural population. Obstet Gynecol 79:245–248
Tattoli M, Cagiano R, Gaetani S, Ghiglieri V, Giustino A, Mereu G, Trabace L, Cuomo V (2001) Neurofunctional effects of developmental alcohol exposure in alcohol-preferring and alcohol-nonpreferring rats. Neuropsychopharmacology 24:691–705
Tournier M, Sorbara F, Gindre C, Swendsen JD, Verdoux H (2003) Cannabis use and anxiety in daily life: a naturalistic investigation in a non-clinical population. Psychiatry Res 118:1–8
Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM (1998) Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83:393–411
Van den Berg CL, Pijlman FT, Koning HA, Diergaarde L, Van Ree JM, Spruijt BM (1999) Isolation changes the incentive value of sucrose and social behaviour in juvenile and adult rats. Behav Brain Res 106:133–142
Vanderschuren LJ, Niesink RJ, Van Ree JM (1997) The neurobiology of social play behavior in rats. Neurosci Biobehav Rev 21:309–326
Wachtel SR, ElSohly MA, Ross SA, Ambre J, de Wit H (2002) Comparison of the subjective effects of delta(9)-tetrahydrocannabinol and marijuana in humans. Psychopharmacology (Berl) 161:331–339
Witkin JM, Tzavara ET, Nomikos GG (2005) A role for cannabinoid CB1 receptors in mood and anxiety disorders. Behav Pharmacol 16:315–331
Acknowledgments
We thank Dr. L.J.M.J. Vanderschuren for critical reading of the manuscript and valuable suggestions and Daniela Valeri, Angela Saraceno, and Alessandra Sordi for technical help. This study was supported by grants PRIN 2005 (to M.R.C.), and FIRB 2006 (to V.C.) from Ministero dell’Università e della Ricerca Scientifica-Italy.
Author information
Authors and Affiliations
Corresponding author
Additional information
Trezza and Campolongo contributed equally to the present study.
Rights and permissions
About this article
Cite this article
Trezza, V., Campolongo, P., Cassano, T. et al. Effects of perinatal exposure to delta-9-tetrahydrocannabinol on the emotional reactivity of the offspring: a longitudinal behavioral study in Wistar rats. Psychopharmacology 198, 529–537 (2008). https://doi.org/10.1007/s00213-008-1162-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-008-1162-3