Abstract
Rationale
Pathological impulsivity is a prominent feature in several psychiatric disorders, but detailed understanding of the specific neuronal processes underlying impulsive behavior is as yet lacking.
Objectives
As recent findings have suggested involvement of the brain cannabinoid system in impulsivity, the present study aimed at further elucidating the role of cannabinoid CB1 receptor activation in distinct measures of impulsive behavior.
Materials and methods
The effects of the selective cannabinoid CB1 receptor antagonist, rimonabant (SR141716A) and agonist WIN55,212-2 were tested in various measures of impulsive behavior, namely, inhibitory control in a five-choice serial reaction time task (5-CSRTT), impulsive choice in a delayed reward paradigm, and response inhibition in a stop-signal paradigm.
Results
In the 5-CSRTT, SR141716A dose-dependently improved inhibitory control by decreasing the number of premature responses. Furthermore, SR141716A slightly improved attentional function, increased correct response latency, but did not affect other parameters. The CB1 receptor agonist WIN55,212-2 did not change inhibitory control in the 5-CSRTT and only increased response latencies and errors of omissions. Coadministration of WIN55,212-2 prevented the effects of SR141716A on inhibitory control in the 5-CSRTT. Impulsive choice and response inhibition were not affected by SR141716A at any dose, whereas WIN55,212-2 slightly impaired response inhibition but did not change impulsive choice.
Conclusions
The present data suggest that particularly the endocannabinoid system seems involved in some measures of impulsivity and provides further evidence for the existence of distinct forms of impulsivity that can be pharmacologically dissociated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite its relative recent discovery, the central endocannabinoid system has been implicated in a variety of behaviors such as food intake (for review, see Di Marzo and Matias 2005) and nociception (for review, see Cravatt and Lichtman 2004), and in mediating the reinforcing properties of drugs of abuse (for review, see De Vries and Schoffelmeer 2005). In addition, the endocannabinoid system has been shown to play an important role in various cognitive processes. In this respect, memory encoding, retrieval, and extinction processes have received most interest (e.g., Marsicano et al. 2002; Takahashi et al. 2005; Varvel et al. 2005; for review, see Lichtman et al. 2002), presumably due to the high expression level of CB1 receptors in the hippocampal formation (Egertova and Elphick 2000; Tsou et al. 1998). High densities of CB1 receptors are also present in frontal cortical and striatal regions (Egertova and Elphick 2000; Tsou et al. 1998), suggesting involvement of the endocannabinoid system in executive functions that appear to be largely controlled by frontal corticostriatal systems (for review, see Miller and Cohen 2001). Indeed, several clinical and preclinical observations have demonstrated that Δ9-tetrahydrocannabinol (THC), the principle active cannabinoid of Cannabis sativa and other synthetic cannabimimetics impair selective attention (Arguello and Jentsch 2004; Solowij et al. 1995; Verrico et al. 2004) and behavioral flexibility (Egerton et al. 2005; Hill et al. 2006), alter time estimation (Han and Robinson 2001, McDonald et al. 2003), and impair working memory (Ilan et al. 2004; Jentsch et al. 1997). Nonetheless, to date, little is known about the involvement of the endocannabinoid system in other executive functions such as inhibitory control processes subserving impulsivity.
Pathological levels of impulsive behavior are important features in attention-deficit/hyperactivity disorder, substance-related disorders, bipolar disorders and personality disorders (American Psychiatric Association 2000). Further elucidating the neurobiological basis of impulsivity may therefore enhance our understanding of these psychiatric disorders. It is becoming increasingly clear, however, that the concept impulsivity is multifaceted and covers various distinct and independent measures. These measures range from poor inhibitory control (impulsive action) to probability and delay aversion or impulsive choice (Barkley 1997; Evenden 1999; Moeller et al. 2001). Recent studies have implicated CB1 receptors in some of these measures of impulsivity. For instance, it has been shown that acute THC impairs response inhibition in healthy volunteers, whereas time estimation and impulsive choice were not affected (McDonald et al. 2003). On the other hand, it has been demonstrated more recently that marijuana acutely increases risk taking in volunteers (Lane et al. 2005). Collectively, these data suggest a role of the cannabinoid system in impulsivity, although its precise role therein is still unclear.
The present experiments were aimed at further elucidating the importance of cannabinoid CB1 receptor activation on distinct measures of impulsivity. To this end, we tested the effects of the potent and selective CB1 receptor antagonist rimonabant (SR141716A; Rinaldi-Carmona et al. 1994) and agonist WIN55,212-2 (D’Ambra et al. 1992) on impulsive behavior in various operant paradigms measuring different and presumably independent aspects of impulsivity (for review, see Winstanley et al. 2006), namely, (1) the five-choice serial reaction time task to measure inhibitory control; (2) the delayed reward paradigm to measure impulsive choice, and (3) the stop-signal paradigm to measure response inhibition.
Materials and methods
Subjects
In total, 48 male Wistar rats were obtained from Harlan CPB (Horst, The Netherlands). At the start of the experiments, animals were 12 weeks old, weighed approximately 250 g, and were housed in pairs in macrolon cages (42.5 × 26.6 × 18.5 cm; l × w × h) under a reversed 12 h light/dark cycle (lights on at 7:00 p.m.) at controlled room temperature (21 ± 2°C) and relative humidity of 60 ± 15%. Animals were maintained at approximately 90% of their free-feeding weight, starting 1 week before the beginning of the experiments by restricting the amount of standard rodent food pellets (Harlan Teklad Global Diet, Blackthorn, UK). Water was available ad libitum throughout the entire experiment. All experiments were conducted with the approval of the animal ethical committee of the Vrije Universiteit, Amsterdam, The Netherlands.
Apparatus
Experiments were conducted in 12 identical rat five-hole nose poke operant chambers with stainless steel grid floors (MED-NPW-5L, Med Associates, St. Albans, VT, USA) housed in sound-insulating and ventilated cubicles. Set in the curved wall of each box was an array of five circular holes, 2.54 cm in diameter, 2.2 cm deep, and 2.25 cm above floor level. Each hole was equipped with an infrared detector located across each nose poke unit 1.0 cm from the front, and a yellow LED stimulus light (6.4 mm in diameter). Rodent food pellets (45 mg, Formula P, Research Diets, New Brunswick, NJ, USA) could be delivered at the opposite wall via a dispenser. In addition, the chamber could be illuminated by a white houselight, and sound stimuli were generated using a programmable audio generator (ANL-926, Med Associates). A computer equipped with MED-PC version 1.17 (Med Associates) controlled experimental sessions and recorded data. Animals were tested once daily from Monday until Friday, during the dark phase of the light/dark cycle.
Behavioral procedures
Separate groups of n = 16 animals were trained for each different paradigm, and for all procedures, a similar habituation and magazine training protocol was used. This protocol consisted of a habituation exposure to the boxes for 20 min with the houselight on and the food cup containing three food pellets for two consecutive sessions. Subsequently, in the next two sessions, in total, 100 pellets were delivered with an average delay of 15 s, to allow the animals to associate the sound of pellet delivery with reward.
Five-choice serial reaction time task
A more detailed description of training in the 5-CSRTT in our laboratory has been reported previously (Van Gaalen et al. 2006a). In short, rats were trained 5 days per week to detect and respond to a brief visual stimulus in one of five holes to obtain a food reward. Each session terminated after 100 trials or 30 min, whichever occurred first. Initially the duration of this stimulus was 32 s and was gradually decreased to 1 s over sessions until animals reached stable baseline performance (accuracy >80% correct choice and <20% errors of omission). Responding during stimulus presentation or within the limited hold (LH) period of 2 s was counted as a correct response. Incorrect, premature responses and errors of omission (no responses or a response after the LH) did not lead to the delivery of a food reward and resulted in a 5-s time-out period during which the houselight was extinguished, whereas perseverative responses, i.e., repeated responding during the presentation of the stimulus, were measured but did not have any programmed consequences. Two different measures of inhibitory control were measured, namely, (1) the number of premature responses before the onset of the visual stimulus, reflecting aspects of loss of inhibitory control and (2) the number of perseverative responses into the stimulus hole after correct choice, presumably measuring aspects of compulsive behavior. In addition, the following other behavioral parameters were measured that reflect task performance, namely, (3) accurate choice, i.e., percentage correct responses calculated as [number correct trials / (correct + incorrect trials)] × 100; (4) latency to make a correct choice, i.e., the mean time between stimulus onset and nose poke in the illuminated hole; (5) omission errors, i.e., the number of omitted trials during a session; and (6) feeder latency, i.e., the latency to collect a pellet following correct choice.
Delayed-reward paradigm
In addition to the 5-CSRTT, the delayed reward paradigm, as employed in our laboratory, has also been described more elaborately (Van Gaalen et al. 2006b). Briefly, rats were trained 5 days per week in this paradigm. In the final stages of training and during drug testing, a session was divided into 5 blocks of 12 trials, each block starting with 2 forced trials during which, after initiating the trial through a nose poke into the central hole, either the left hole or the right hole was illuminated in a counterbalanced fashion. In the next ten trials, the animals had a free choice, and both the left and right hole were illuminated. Poking into one position resulted in the immediate delivery of a small reinforcer (one food pellet), whereas a nose poke into the other position resulted in the delivery of a large, but delayed, reinforcer (four food pellets). If an animal did not make a response during this choice phase within 10 s, an intertrial interval was initiated, and the trial was counted as an omission. The position associated with the small and large reinforcer was always the same for each individual and counterbalanced for the group. The delay for the large reinforcer progressively increased within a session per block of 12 trials as follows: 0, 10, 20, 40, and 60 s. Responding into non-illuminated holes during the test was recorded, but had no programmed consequences. The behavioral measure to assess task performance, i.e., the percentage preference for the large reinforcer as a function of delay, was calculated as: the number of choices for the large reinforcer / (number choices large + small reinforcers) × 100. In addition, we calculated the total number of omitted choice trials within a session.
Stop-signal paradigm
Shaping
During initial shaping for two consecutive sessions, both the middle nose poke hole and the hole immediately adjacent to the right or left were illuminated (counterbalanced for all subjects). A nose poke into either one of the two active holes extinguished the visual stimuli in both holes and resulted in delivery of a pellet. After an intertrial interval of 10 s, the next trial started. Nose poking within this intertrial interval period did not have any programmed consequences. A session ended when the rat had earned 100 pellets or after 30 min, whichever occurred first.
Shaping: go trials
During the next phase, only the stimulus light in the middle nose poke hole was illuminated (start stimulus). A response into the active middle hole switched off the stimulus light and was followed by the illumination of the stimulus light (go stimulus) in the hole immediately adjacent to the left or right. A nose poke into this illuminated hole switched off the stimulus light and resulted in the delivery of a pellet. After an intertrial interval of 10 s, the next trial started. Responding in the start stimulus hole during presentation of the go stimulus was counted as perseverative start pokes, whereas prestimulus responses into the go stimulus hole resulted in a timeout period of 5 s. Subsequently, the response requirements into the start stimulus hole before onset of a go stimulus were varied into a variable ratio 2 schedule (VR2, i.e., either FR1, FR2, or FR3) to avoid the development of a prepotent response pattern from start stimulus to go stimulus hole and to ensure that animals waited until the appearance of a go stimulus. During this phase, rats were trained until they reliably completed 200 successful go trials. Following this phase, a LH period was introduced for the go stimulus and only during this period was the go stimulus present. Initially, the LH was set at 5 s, and in subsequent sessions, was individually titrated to meet performance criterion of 80% successful hits and <20% prestimulus responses. Omissions of a go stimulus response within the LH resulted in a 5-s time-out period, during which both the houselight and stimulus light were turned off. From initial shaping to criterion performance in this phase, 26 sessions were required.
Shaping: introduction stop signal
During the final training phase, a stop signal was introduced in 25% of all trials. Initially, this stop signal (duration, 50 ms; frequency, 4,500 Hz; intensity 80 dB) was contingent with the appearance of the go signal. Responding during the onset of the stop signal or during the LH immediately extinguished the go stimulus and houselight, turned off the stop signal, and was followed by a 5-s time-out. In contrast, if the animal successfully refrained from responding during a stop trial, a pellet was delivered. Initially, the LH during stop and go trials were equal; however, when performance during stop trials was below 80% successfully inhibited stop trials, the LH during stop trials was lowered over sessions in steps of 100–200 ms until animals improved performance. Subsequently, the LH was then gradually increased in these individuals over sessions until the LH during both go and stop trials were equal. As soon as animals reached the criterion of approximately 90% successfully inhibited stop trials, delays for the onset of the stop signal were introduced. The stop-signal delays (SSD) were presented in a pseudorandom order, and to compensate for differences between rats, SSDs were based on each individual rats’ mean reaction time on go trials in the preceding drug-free training session. SSDs were calculated as follows: mRT minus either 25, 50, 100, 200, or 400 ms. In addition, an equal amount of zero delays were presented during sessions. Drug testing commenced upon stable baseline performance for at least five consecutive sessions, i.e., 80% accuracy during go trials and a significant SSD-dependent decrease in correctly inhibited stop trials. It took 30 sessions before animals reached stable baseline performance after the introduction of the stop signal; therefore, from initial shaping to stable baseline performance in the stop-signal paradigm, in total, 56 sessions were required.
Stop-signal paradigm: estimation stop-signal reaction time and correction for omissions during go trials
Calculations to estimate the stop-signal reaction times (SSRT) and a correction for omission errors were adapted from Logan (1994) and Solanto et al. (2001). For estimating the SSRT, data of the three SSDs of 200, 100, and 50 ms were used, as the probability of correct inhibition on these intervals was within the range of 0.2 < p < 0.8, and thus, most informative for estimating SSRT (Band et al. 2003). For each of the three intervals, the probability of responding was calculated including a correction for nonresponses based on the number of omissions during the go trials, the latter, as omissions cannot be distinguished from successful inhibitions during stop trials. The following formula, adapted from Solanto et al. (2001) was used for these calculations:
where x is the number of stop-signal trials at each delay interval; correct inhibitions are the number of correctly inhibited trials, and y is the probability of omissions during the go trials within the entire session. To calculate SSRTs, reaction times on all go trials were rank ordered. From this list with RTs, the “nth” RT was taken, where “n“ was obtained by multiplying the total number of go trials by the probability of responding for a particular SSD. This RT value approximates the latency between onset of the go stimulus and completion of the stopping process. The SSRT for each interval is then obtained by subtracting the SSD interval from this RT. The average estimated SSRT that is used for the analyses in the present study is calculated by taking the mean of each SSRT at the three SSDs (200, 100, and 50 ms).
Drugs
SR141716A was generated and kindly donated by Solvay Pharmaceuticals (Weesp, The Netherlands), whereas WIN55,212-2 was purchased from Tocris Biosciences (Bristol, United Kingdom). Both SR141716A and WIN55,212-2 were dissolved as described previously in a mixture of ethanol, Tween80, and sterile saline (ratio 1:1:18; cf. De Vries et al. 2001). In all experiments, SR141716A was injected 30 min before testing, whereas WIN55,212-2 was injected 20 min before testing. In all paradigms, the order of testing the drugs was (1) SR141716A and (2) WIN55,212-2. The drug combination was only performed in the five-choice serial reaction time task and followed the studies with SR141716A and WIN55,212-2. Drugs were freshly prepared each day before testing and intraperitoneally injected in a volume of 1 ml/kg bodyweight according to a Latin square design for both the dose–response studies and the drug combination on Tuesdays and Fridays, with baseline training sessions on the other weekdays.
Statistical analyses
Data were subjected to repeated measures analysis of variance (ANOVA) with drug dose (all paradigms), delay to large reinforcer (delayed reward paradigm), and stop-signal delay (stop-signal paradigm) as within subjects variables using the Statistical Package for the Social Sciences version 11 (SPSS, Chicago, IL, USA).
The homogeneity of variance across groups was determined using Mauchly’s tests for equal variances, and in case of violation of homogeneity, corrected, and therefore, more conservative Huynh–Feldt probability values were used for subsequent analyses. In the stop-signal paradigm, some further exploratory analyses were performed between mean go reaction times and the limited hold period using a bivariate Pearson correlation. In addition, the estimated SSRT data were also subjected to a median split analysis to assess whether SR141716A or WIN55,212-2 had differential effects on response inhibition in individuals with relatively “fast” or “slow” stopping abilities. In case of statistically significant main effects, further post hoc comparisons were conducted using Student–Newman–Keuls Tests. The level of probability for statistically significant effects was set at 0.05.
Results
Effects of SR141716A and WIN55,212-2 on measures of inhibitory response control in the five-choice serial reaction time task
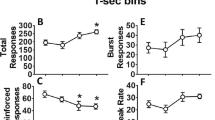
The number of premature responses, a measure of inhibitory control reflecting impulsive behavior, was dose dependently decreased by SR141716A [Fig. 1a; F 3,45 = 12.24, p < 0.001], and further post hoc analyses revealed that all doses significantly lowered premature responding compared with vehicle, whereas the highest dose (3.0 mg/kg) even further lowered the number of premature responses compared with 1.0 mg/kg SR141716A. In contrast, perseverative responding after correct choice, a different measure of inhibitory control reflecting compulsive behavior, was not affected at any dose [Fig. 1b; F 3,45 = 1.84, p = 0.15]. Attentional function was improved by SR141716A, and further analyses revealed that only 0.3 mg/kg SR141716A significantly increased the percentageof accurate choice from approximately 77% under vehicle conditions to 82% as shown in Table 1 [F 3,45 = 3.43, p = 0.025]. In addition, a marginal, but significant, increase in the latency to make a correct choice was also detected, and further comparisons showed that only the high dose of 3.0 mg/kg significantly slowed correct response reaction time [Table 1; F 3,45 = 4.53, p = 0.007]. The latency to collect a food reward and errors of omission, however, were not affected at any dose [Table 1; feeder latency: F 3,45 = 0.39, p = 0.65 and omissions: F 3,45 = 1.30, p = 0.29].
WIN55,212-2 did neither change the number of premature responses nor the number of perseverative responses after correct choice [Fig. 1c and d; premature responses: F 3,45 = 1.02, p = 0.39 and perseverative responses: F3,45 = 1.64, p = 0.21; respectively]. Furthermore, as indicated in Table 1, WIN55,212-2 did not change accurate choice or the latency to collect a pellet after correct choice [accurate choice: F 3,45 = 0.72, p = 0.54 and feeder latency: F 3,45 = 3.19, p = 0.052]. In contrast, errors of omission were increased, and latencies to make a correct choice were lengthened by WIN55,212-2 [omissions: F 3,45 = 7.44, p = 0.002 and correct response latency: F 3,45 = 6.59, p = 0.004].
As shown in Fig. 2, the decrements in premature responding by 3.0 mg/kg SR141716A were prevented in the presence of WIN55,212-2 at a dose of 1.0 mg/kg [F 3,45 = 7.33, p < 0.001]. In addition, this selected dose of WIN55,212-2, by itself, did not affect inhibitory control as indicated by the absence of an effect of this dose on the number of premature responses.
SR141716A and WIN55,212-2 do not affect decision making in the delayed reward paradigm
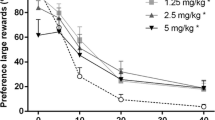
Stable baseline performance on the delayed reward paradigm occurred after approximately 30 training sessions on a full delay range (0, 10, 20, 40, and 60 s), and therefore, we commenced drug testing from session 35 onwards. A clear, highly significant delay-dependent decrement in the percentage preference for the large reinforcer was observed [Fig. 3; delay: F 4,60=270.22, p < 0.001]. Nonetheless, SR141716A did not shift the preference for a large reinforcer over delays at any of the tested doses [dose: F 3,45 = 1.56, p = 0.21 and dose × delay: F 12,180 = 1.24, p = 0.29]. In addition, SR141716A also did not change the total numbers of omitted choice trials, i.e., the failures to start a trial during the choice phase [dose: F 3,45 = 3.10, p = 0.052].
In the WIN55,212-2 experiments, at the highest dose, in total, five animals omitted all choice trials of some delays, and therefore, were excluded from all analyses of the delay discounting data. Similar to SR141716A, increasing the delay highly significantly shifted the preference from large to the small reinforcer [Fig. 4; delay: F 4,40 = 179.06, p < 0.001]. Although there was no overall effect of WIN55,212-2 on decision making [dose: F 3,30 = 0.68, p = 0.57], there was a dose by delay interaction effect suggesting a shift in preference for the large reinforcer over delays [dose × delay: F 12,120 = 2.61, p = 0.011]. Nonetheless, further post hoc comparisons revealed no differences between vehicle and any of the other doses. The number of omitted choice trials was not affected by WIN55,212-2 [dose: F 3,30 = 1.61, p = 0.21].
Effects of SR141716A on response inhibition as measured in the stop-signal paradigm. Data are depicted as mean (±SEM) percentage of correctly inhibited stop trials with varying SSDs before the mean go RT (a), go reaction times (b) and estimated stop signal reaction times from SSDs 200, 100, and 50 ms (c). **p < 0.005 vs vehicle
Effects of SR141716A and WIN55,212-2 on response inhibition in the stop-signal paradigm
Under baseline conditions in the stop-signal paradigm, there was stable individual variation in mean reaction times during go trials over consecutive sessions ranging from 290 to 470 ms (mean: 365 ms; standard deviation: 58 ms). Likewise, the limited hold periods during go and stop trials also stably varied and ranged from 400 to 1,100 ms (mean: 594 ms; SD: 167 ms). A highly significant positive correlation indicated that individuals with “shorter” limited hold periods also displayed shorter mean go reaction times compared to individuals with “longer” limited hold periods that were slower in go reaction speed [r = 0.85, p < 0.001].
Response inhibition, as displayed in the inhibition function curve in Fig. 4a, was not affected by SR141716A at any of the tested doses [dose: F 3,45 = 1.28, p = 0.29 and dose × SSD: F 12,180 = 1.25, p = 0.26]; however, the percentage of correctly inhibited stop trials significantly declined with decreasing stop-signal delays [SSD: F 4,60 = 39.24, p < 0.001]. SR141716A did slow mean reaction times during go trials by approximately 30 ms [Fig. 4b; dose: F 3,45 = 7.93, p = 0.001], and further analyses indicated that both the 1.0 and 3.0 mg/kg dose significantly slowed reaction times compared with vehicle. Nonetheless, the number of omissions during go trials were not significantly affected [mean ± SEM: vehicle = 16.2 ± 3.0; 0.3 mg/kg = 18.1 ± 1.2; 1.0 mg/kg = 17.7 ± 1.7 and 3.0 mg/kg = 23.3 ± 2.6; dose: F 3,45 = 1.76, p = 0.17]. Lastly, the average estimated SSRT across three stop-signal delays, putatively reflecting response inhibition, was not significantly changed by SR141716A at any dose [Fig. 4c; dose: F 3,45 = 1.47, p = 0.24]. Furthermore, a median split analysis (median SSRT: 238 ms) did not reveal differential effects of SR141716A on the estimated SSRT in individuals with relatively “fast” vs “slow” stopping abilities [slow vs fast stopper: F 1,14 = 9.54, p = 0.008; dose: F 3,42 = 1.04, p = 0.38 and dose × slow vs fast stopper: F 3,42 = 2.48, p = 0.074].
In the WIN55,212-2 experiments, at the highest dose, in total, four animals did not start any go trials and were therefore excluded from all stop-signal data analyses. Response inhibition, as displayed in the inhibition function curve in Fig. 5a, was affected by WIN55,212-2 [SSD: F 4,44 = 23.51, p < 0.001; dose: F 3,33 = 5.12, p = 0.005 and dose × SSD: F 12,132 = 1.11, p = 0.36], and further comparisons revealed that 0.3 mg/kg WIN55,212-2 significantly deteriorated the percentage of correct inhibition compared to vehicle. Mean reaction times during go trials were slowed by WIN55,212-2 [Fig. 5b; dose: F 3,33 = 15.07, p < 0.001], and further analyses indicated that 1.0 and 3.0 mg/kg WIN55,212-2 significantly slowed mean reaction times compared to vehicle. Nonetheless, the number of omissions during go trials were not significantly affected [mean ± SEM: vehicle = 14.8 ± 1.9; 0.3 mg/kg = 23.2 ± 4.8; 1.0 mg/kg = 26.1 ± 5.4 and 3.0 mg/kg = 34.7 ± 7.0; dose: F 3,33 = 3.06, p = 0.073]. Lastly, the average estimated SSRT across three stop-signal delays, putatively reflecting response inhibition, was not significantly changed by WIN55,212-2 at any dose [Fig. 5c; dose: F 3,33 = 2.06, p = 0.13]. Furthermore, a median split analysis (median SSRT: 227 ms) did not reveal differential effects of WIN55,212-2 on the estimated SSRT in individuals with relatively “fast” vs “slow” stopping abilities [slow vs fast stopper: F 1,10 = 9.57, p = 0.011; dose: F 3,30 = 2.12, p = 0.12 and dose × slow vs fast stopper: F 3,30 = 1.30, p = 0.29].
Effects of WIN55,212-2 on response inhibition as measured in the stop-signal paradigm. Data are depicted as mean (±SEM) percentage of correctly inhibited stop trials with varying SSDs before the mean go reaction times (a), go RTs (b) and estimated stop signal reaction times from SSDs 200, 100, and 50 ms (c). *p < 0.05 and **p < 0.005 vs vehicle
Discussion
To our knowledge, this is the first report demonstrating behavioral effects of a CB1 receptor agonist and antagonist on independent measures of impulsivity. Thus far, evidence pointing towards cannabinoid involvement in impulsivity mainly originates from studies in which effects of THC or marijuana were tested in human volunteers (Lane et al. 2005; McDonald et al. 2003). Our data obtained in the 5-CSRTT strongly suggest that inhibitory control is modulated by an endogenous cannabinoid tone, as premature responding was dose dependently decreased by SR141716A. This notion is further supported by the observation that in the presence of WIN55,212-2, the effects of 3.0 mg/kg SR141716A on premature responding were no longer observed. Furthermore, the finding that the CB1 receptor agonist WIN55,212-2, by itself, did not impair inhibitory control by increasing the number of premature responses is in keeping with previous findings (Arguello and Jentsch 2004). It should be noted though, that in the study by Arguello and Jentsch (2004), SR141716A (dose range: 0.1–1.0 mg/kg), by itself, did not change premature responding in a lateralized reaction time task, thereby, contrasting our findings. Nonetheless, differences in baseline performance may have contributed to the discrepancy in findings, as premature responding in well-trained rats in the task by Arguello and Jentsch (2004) was low (approximately 3 per session) compared to premature responding in well-trained rats in the present study (approximately 22 per session). Presumably, a floor effect may have masked the effects of SR141716A on premature responding in the lateralized reaction time task.
Together, our data indicate that performance in the 5-CSRTT is associated with profound occupation of CB1 receptors by endogenously released cannabinoids. As might then be expected, the endocannabinoid uptake inhibitor AM404 did not affect inhibitory response control in the 5-CSRTT (unpublished data). In contrast to premature responding, perseverative responding after correct choice, a different measure of inhibitory control that putatively reflects compulsive behavior (Robbins 2002), was neither altered upon activation nor blockade of CB1 receptors. This finding further underscores the dissociation between premature and perseverative responding in the 5-CSRTT that has been shown to have a neuroanatomical basis as well (Chudasama et al. 2003).
The endocannabinoid system interacts with many other central neurotransmitters systems including the cholinergic, GABAergic, glutamatergic, and opioid systems (Schlicker and Kathmann 2001; Schoffelmeer et al. 2006). Moreover, cannabimimetics have been shown to indirectly modulate the release of dopamine and glutamate in corticostriatal regions (e.g., Cheer et al. 2004; Szabo et al. 1999; Tanda et al. 1997; Xi et al. 2006), most notably in the nucleus accumbens, and particularly, these effects have been linked to the involvement of the cannabinoid system in addiction (for review, see De Vries and Schoffelmeer 2005). Although it is beyond the scope of the present study and additional experiments are required to substantiate this, one might speculate that the present findings are explained by these modulatory effects of the cannabinoid system on mesolimbic dopamine release, as inhibitory control processes in the 5-CSRTT have been shown to depend upon dopamine receptor activation within the nucleus accumbens (Cole and Robbins 1987; Pattij et al. 2007). On the other hand, explanations for the beneficial effects of SR141716A on inhibitory control may be due to the role of the endocannabinoid system in feeding behavior (for review, see Di Marzo and Matias 2005). Accordingly, it has been demonstrated that SR141716A reduces intake of normal and palatable food (e.g., Arnone et al. 1997; Freedland et al. 2000; Thornton-Jones et al. 2005) and the motivation to obtain food (Solinas and Goldberg 2005). Nonetheless, despite these reported effects of SR141716A on food intake, anorexic effects of this compound seem unlikely to explain the current data, as both primary indices of food-motivated behavior in the 5-CSRTT, namely, errors of omission and feeder response latencies, were not changed.
It is interesting to note that we also observed a moderate beneficial effect of SR141716A on visuospatial attention, as it increased the level of accurate choice at 0.3 mg/kg. Likewise, it has been shown that SR141716A improves social recognition and spatial and aversive memory at comparable dose ranges between 0.3 and 3.0 mg/kg (Lichtman 2000; Takahashi et al. 2005; Terranova et al. 1996; Wolff and Leander 2003). Although the mechanisms responsible for the beneficial effects of disrupting endocannabinoid signaling on mnemonic and attentional processes need to be elucidated further, it is interesting to note that SR141716A increases, among others, cholinergic neurotransmission in the medial prefrontal cortex in vivo (Tzavara et al. 2003). This finding may be particularly relevant for attentional function, as performance in the 5-CSRTT has been shown to be accompanied by increments in acetylcholine release in the medial prefrontal cortex (Passetti et al. 2000), whereas decrements in acetylcholine release in this brain region have been shown to correlate with poor visuospatial attention (McGaughy et al. 2002).
A different measure of impulsivity we studied was impulsive choice in the delayed reward paradigm. Conceptually, impulsive choice differs from inhibitory control in the 5-CSRTT and rather reflects a cognitive decision-making process, as rats have to weigh the immediate vs delayed outcomes of their behavior. Impulsive choice then is reflected in insensitivity towards the delayed larger reward and a preference for the small immediate reward. In the present experiments, we observed that neither SR141716A nor WIN55,212-2 did have any effects on impulsive choice. In accordance with these findings, it has been shown recently that THC did not change delay and probability discounting in human volunteers (McDonald et al. 2003), and together, these data suggest that the (endo)cannabinoid system is not critically involved in impulsive choice. Our observations do contrast with previous findings indicating that marijuana elevates risk-taking behavior in volunteers (Lane et al. 2005). Risk taking, however, differs from delay and probability aversion in that it is most likely to occur when the behavioral options may result in losses or have aversive consequences (Rachlin et al. 1986). In the delayed reward paradigm and the paradigm used by McDonald et al. (2003), subjects could only win and not lose reward (food or money, respectively), whereas only the magnitude of the reward depended upon choice. It is therefore possible, that these procedural and conceptual differences explain the discrepancy in findings, and furthermore, that delay aversion and risk-taking processes are differentially regulated by the (endo)cannabinoid system.
While response inhibition has been shown to be impaired in human volunteers after THC administration (McDonald et al. 2003), neither disruption of endocannabinoid signaling nor administration of a CB1 receptor agonist had clear observable behavioral effects on stop-signal task performance. In agreement with the assumption of a “race model” between “go” and “stop” processes (Logan 1994) and previous stop-signal data in rats (Eagle and Robbins 2003a, b), the probability of successfully inhibiting a response during a stop trial decreased in rats when the onset of the stop signal was delayed in time. Although SR141716A did not shift this inhibition curve as a function of increasing stop-signal delays, the low dose WIN55,212-2 (0.3 mg/kg) significantly deteriorated the ability to inhibit responding with increasing stop-signal delays. However, the primary parameter in this paradigm, the average estimated stop-signal reaction time, or simply put, speed of stopping, was not changed by any dose of SR141716A or WIN55,212-2. In addition, median split analyses revealed no differential drug effects in individuals with “slow” vs “fast” stopping abilities as has been reported previously for the effects of d-amphetamine on stopping speed in both rats and humans (De Wit et al. 2002; Feola et al. 2000). Collectively, our findings suggest that response inhibition processes, as measured in the stop-signal paradigm, are not under control of CB1 receptors. In the present study, the speed of stopping was estimated from separate SSRTs obtained from three stop-signal delays (200, 100, and 50 ms before mean go RT), and consistent with this estimation method, there was some variability in the SSRTs depending on the delay (Band et al. 2003; Logan 1994), with shorter stop-signal delays resulting in SSRTs of approximately 140 ms and longer delays resulting in SSRTs of approximately 300 ms. However, the variability in SSRTs over delays was similar across all doses of both drugs (data not shown), thus, ruling out the possibility that this variability may have masked effects of SR141716A or WIN55,212-2 on response inhibition. In contrast to the measures of response inhibition, reaction times during performance on go trials were slowed by both compounds in line with their effects in the 5-CSRTT. With regard to these effects of SR141716A, in the absence of changes on omission errors in both the 5-CSRTT and the stop signal paradigm, they cannot be solely interpreted in terms of motor effects. Rather, the minor but significant increase in response latencies of approximately 30 ms in both paradigms (Table 1 and Fig. 4b) may indicate changes in information processing speed induced by blockade of endocannabinoid signaling. However, this notion is not confirmed by previous sensorimotor gating data (Mansbach et al. 1996; Martin et al. 2003). In contrast, the effects of 1.0 and 3.0 mg/kg WIN55,212-2 on reaction times in the stop-signal paradigm may in part be secondary to changes in locomotor activity, as these doses also increased the number of omissions in the 5-CSRTT.
Remarkably, although both the stop-signal paradigm and 5-CSRTT measure aspects of impulsive action, only inhibitory control in the 5-CSRTT was improved by SR141716A. Impulsive action in the latter paradigm is mainly measured as the inability to inhibit inappropriate (premature) responses, whereas in the stop-signal paradigm impulsive action is reflected in the inability to inhibit ongoing behavior, i.e., the inability to stop a behavioral response that has just been initiated. Our observations support the notion of different and separable forms of inhibitory control, and moreover, suggest a differential role for the endocannabinoid system therein. Likewise, lesion studies have suggested that different brain regions are involved in inhibitory control measured in either the 5-CSRTT or stop-signal paradigm, as for instance, lesions of the nucleus accumbens or subregions of the medial prefrontal cortex have been shown to impair inhibitory control in the 5-CSRTT (Christakou et al. 2004; Chudasama et al. 2003; Muir et al. 1996) and not in the stop-signal paradigm (Eagle and Robbins 2003b).
In summary, the present study provides evidence for a differential involvement of the endocannabinoid system in independent measures of impulsivity, as SR141716A primarily affected inhibitory control, and neither impulsive choice nor response inhibition, whereas WIN55,212-2 only slightly affected response inhibition. In this regard, the present data add to existing clinical and preclinical evidence demonstrating that distinct measures of impulsivity can be dissociated at a pharmacological and neuroanatomical level (e.g., Chudasama et al. 2003; De Wit et al. 2002; McDonald et al. 2003; Winstanley et al. 2004). Our findings may be of particular interest with respect to the heterogeneity observed in attention-deficit/hyperactivity disorder (Sonuga-Barke 2002) and suggest that possible novel pharmacotherapies targeted at the cannabinoid system may benefit the subtype resulting from poor inhibitory control, but not the motivational style, or delay aversion, subtype.
References
American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders. Fourth Edition, Text Revision. American Psychiatric Association, Washington
Arguello PA, Jentsch JD (2004) Cannabinoid CB1 receptor-mediated impairment of visuospatial attention in the rat. Psychopharmacology 177:141–150
Arnone M, Maruani J, Chaperon F, Thiebot MH, Poncelet M, Soubrie P, Le Fur G (1997) Selective inhibition of sucrose and ethanol intake by SR141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology 132:104–106
Band GPH, Van der Molen MW, Logan GD (2003) Horse-race model simulations of the stop-signal procedure. Acta Psychol 112:105–142
Barkley RA (1997) Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull 121:65–94
Cheer JF, Wassum KM, Heien MLAV, Phillips PEM, Wightman RM (2004) Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J Neurosci 24:4393–4400
Christakou A, Robbins TW, Everitt BJ (2004) Prefrontal cortical–ventral striatal interactions involved in affective modulation of attentional performance: implications for corticostriatal circuit function. J Neurosci 24:773–780
Chudasama Y, Passetti F, Rhodes SEV, Lopian D, Desai A, Robbins TW (2003) Dissociable aspects following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res 146:105–119
Cole BJ, Robbins TW (1987) Amphetamine impairs the discriminative performance of rats with dorsal noradrenergic bundle lesions on a 5-choice serial reaction time task: new evidence for central dopaminergic–noradrenergic interactions. Psychopharmacology 91:458–466
Cravatt BJ, Lichtman AH (2004) The endogenous cannabinoid system and its role in nociceptive behavior. J Neurobiol 61:149–160
D’Ambra TE, Estep KG, Bell MR, Eissenstat MA, Josef KA, Ward SJ, Haycock DA, Baizman ER, Casiano FM, Beglin NC, Chippari SM, Grego JD, Kullnig RK, Daley GT (1992) Conformationally restrained analogues of pravadoline: nanomolar potent, enantioselective, (aminoalkyl)indole agonists of the cannabinoid receptor. J Med Chem 35:124–135
De Vries TJ, Schoffelmeer ANM (2005) Cannabinoid CB1 receptors control conditioned drug seeking. Trends Pharmacol Sci 26:420–426
De Vries TJ, Shaham Y, Homberg J, Crombag H, Schuurman K, Dieben J, Vanderschuren LJMJ, Schoffelmeer ANM (2001) A cannabinoid mechanism in relapse to cocaine seeking. Nat Med 7:1151–1154
De Wit H, Enggasser JL, Richards JB (2002) Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology 27:813–825
Di Marzo V, Matias I (2005) Endocannabinoid control of food intake and energy balance. Nat Neurosci 8:585–589
Eagle DM, Robbins TW (2003a) Inhibitory control in rats performing a stop-signal reaction-time task: effects of lesions of the medial striatum and d-amphetamine. Behav Neurosci 117:1302–1317
Eagle DM, Robbins TW (2003b) Lesions of the medial prefrontal cortex or nucleus accumbens core do not impair inhibitory control in rats performing a stop-signal reaction time task. Behav Brain Res 146:131–144
Egerton A, Brett RR, Pratt JA (2005) Acute delta9-tetrahydrocannabinol-induced deficits in reversal learning: neural correlates of affective inflexibility. Neuropsychopharmacology 30:1895–1905
Egertova M, Elphick MR (2000) Localisation of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB1. J Comp Neurol 422:159–171
Evenden JL (1999) Varieties of impulsivity. Psychopharmacology 146:348–361
Feola TW, De Wit H, Richards JB (2000) Effects of d-amphetamine and alcohol on a measure of behavioral inhibition in rats. Behav Neurosci 114:838–848
Freedland CS, Poston JS, Porrino LJ (2000) Effects of SR141716A, a central cannabinoid receptor antagonist, on food-maintained responding. Pharmacol Biochem Behav 67:265–270
Han CJ, Robinson JK (2001) Cannabinoid modulation of time estimation in the rat. Behav Neurosci 115:243–246
Hill MN, Froese LM, Morrish AC, Sun JC, Floresco SB (2006) Alterations in behavioral flexibility by cannabinoid CB1 receptor agonists and antagonists. Psychopharmacology 187(2):245–259
Ilan AB, Smith ME, Gevins A (2004) Effects of marijuana on neurophysiological signals of working and episodic memory. Psychopharmacology 176:214–222
Jentsch JD, Andrusiak E, Tran A, Bowers MB, Roth RH (1997) Delta9-Tetrahydrocannabinol increases prefrontal cortical catecholaminergic utilization and impairs spatial working memory in the rat: blockade of dopaminergic effects with HA966. Neuropsychopharmacology 16:426–432
Lane SD, Cherek DR, Tcheremissine OV, Lieving LM, Pietras CJ (2005) Acute marijuana effects on human risk taking. Neuropsychopharmacology 30:800–809
Lichtman AH (2000) SR 141716A enhances spatial memory as assessed in a radial-arm maze task in rats. Eur J Pharmacol 404:175–179
Lichtman AH, Varvel SA, Martin BR (2002) Endocannabinoids in cognition and dependence. Prostaglandins Leukot Essent Fatty Acids 66:269–285
Logan GD (1994) On the ability to inhibit thought and action: a users’ guide to the stop signal paradigm. In: Dagenbach D, Carr TH (eds) Inhibitory processes in attention, memory, and language. Academic, San Diego, pp 189–239
Mansbach RS, Rovetti CC, Winston EN, Lowe JA (1996) Effects of the cannabinoid CB1 receptor antagonist SR141716A on the behavior of pigeons and rats. Psychopharmacology 124:315–322
Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B (2002) The endogenous cannabinoid system controls extinction of aversive memories. Nature 418:488–489
Martin RS, Secchi RL, Sung E, Lemaire M, Bonhaus DW, Hedley LR, Lowe DA (2003) Effects of cannabinoid receptor ligands on psychosis-relevant behavior models in the rat. Psychopharmacology 165:128–135
McDonald J, Schleifer L, Richards JB, de Wit H (2003) Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology 28:1356–1365
McGaughy J, Dalley JW, Morrison CH, Everitt BJ, Robbins TW (2002) Selective behavioral and neurochemical effects of cholinergic lesions produced by intrabasalis infusions of 192 IgG-Saporin on attentional performance in a five-choice serial reaction time task. J Neurosci 22:1905–1913
Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC (2001) Psychiatric aspects of impulsivity. Am J Psychiatry 158:1783–1793
Miller EK, Cohen JD (2001) An integrative theory of prefrontal cortex function. Annual Rev Neurosci 24:167–202
Muir JL, Everitt BJ, Robbins TW (1996) The cerebral cortex of the rat and visual attentional function: dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cereb Cortex 6:470–481
Passetti F, Dalley JW, O’Connell MT, Everitt BJ, Robbins TW (2000) Increased acetylcholine release in the rat medial prefrontal cortex during performance of a visual attentional task. Eur J Neurosci 12:3051–3058
Pattij T, Janssen MC, Vanderschuren LJ, Schoffelmeer AN, van Gaalen MM (2007) Involvement of dopamine D1 and D2 receptors in the nucleus accumbens core and shell in inhibitory response control. Psychopharmacology 191:587–598
Rachlin H, Logue AW, Gibbon J, Frankel M (1986) Cognition and behavior in studies of choice. Psychol Rev 93:33–45
Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Neliat G, Caput D, Ferrara P, Soubrie P, Breliere JC, Le Fur G (1994) SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett 350:240–244
Robbins TW (2002) The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology 163:362–380
Schlicker E, Kathmann M (2001) Modulation of transmitter release via presynaptic cannabinoid receptors. Trends Pharmacol Sci 22:565–572
Schoffelmeer ANM, Hogenboom F, Wardeh G, De Vries TJ (2006) Interactions between CB1 cannabinoid and ì opioid receptors mediating inhibition of neurotransmitter release in rat nucleus accumbens core. Neuropharmacology 51:773–781
Solanto MV, Abikoff H, Sonuga-Barke E, Schachar R, Logan GD, Wigal T, Hechtman L, Hinshaw S, Turkel E (2001) The ecological validity of delay aversion and response inhibition as measures of impulsivity in AD/HD: a supplement to the NIMH multimodal treatment study of AD/HD. J Abnorm Child Psychol 29:215–228
Solinas M, Goldberg SR (2005) Motivational effects of cannabinoids and opioids on food reinforcement depend on simultaneous activation of cannabinoid and opioid systems. Neuropsychopharmacology 30:2035–2045
Solowij N, Michie PT, Fox AM (1995) Differential impairment of selective attention due to frequency and duration of cannabis use. Biol Psychiatry 37:731–739
Sonuga-Barke EJS (2002) Psychological heterogeneity in AD/HD—a dual pathway model of behaviour and cognition. Behav Brain Res 130:29–36
Szabo B, Muller T, Koch H (1999) Effects of cannabinoids on dopamine release in the corpus striatum and the nucleus accumbens in vitro. J Neurochem 73:1084–1089
Takahashi RN, Pamplona FA, Fernandes MS (2005) The cannabinoid antagonist SR141716A facilitates memory acquisition and consolidation in the mouse elevated T-maze. Neurosci Lett 380:270–275
Tanda G, Pontieri FE, Di Chiara G (1997) Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common μ1 opioid receptor mechanism. Science 276:2048–2050
Terranova JP, Storme JJ, Lafon N, Perio A, Rinaldi-Carmona M, Le Fur G, Soubrie P (1996) Improvement of memory in rodents by the selective CB1 cannabinoid antagonist, SR141716. Psychopharmacology 126:165–172
Thornton-Jones ZD, Vickers SP, Clifton PG (2005) The cannabinoid CB1 receptor antagonist SR141716A reduces appetitive and consummatory responses for food. Psychopharmacology 179:452–460
Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM (1998) Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83:393–411
Tzavara ET, Davis RJ, Perry KW, Li X, Salhoff C, Bymaster FP, Witkin JM, Nomikos GG (2003) The CB1 receptor antagonist SR141716A selectively increases monoaminergic neurotransmission in the medial prefrontal cortex: implications for therapeutic actions. Br J Pharmacol 138:544–553
Van Gaalen MM, Brueggeman RJ, Bronius PFC, Schoffelmeer ANM, Vanderschuren LJMJ (2006a) Behavioral disinhibition requires dopamine receptor activation. Psychopharmacology 187:73–85
Van Gaalen MM, Van Koten R, Schoffelmeer ANM, Vanderschuren LJMJ (2006b) Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biol Psychiatry 60:66–73
Varvel SA, Anum EA, Lichtman AH (2005) Disruption of CB1 receptor signaling impairs extinction of spatial memory in mice. Psychopharmacology 179:863–872
Verrico CD, Jentsch JD, Roth RH, Taylor JR (2004) Repeated, intermittent delta(9) tetrahydrocannabinol administration to rats impairs acquisition and performance of a test of visuospatial divided attention. Neuropsychopharmacology 29:522–529
Winstanley CA, Dalley JW, Theobald DE, Robbins TW (2004) Fractionating impulsivity: contrasting effects of central 5-HT depletion on different measures of impulsive behavior. Neuropsychopharmacology 29:1331–1343
Winstanley CA, Eagle DM, Robbins TW (2006) Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev 26:379–395
Wolff MC, Leander JD (2003) SR141716A, a cannabinoid CB1 receptor antagonist, improves memory in a delayed radial arm maze. Eur J Pharmacol 477:213–217
Xi ZX, Gilbert JG, Peng XQ, Pak AC, Li X, Gardner EL (2006) Cannabinoid CB1 receptor antagonist AM251 inhibits cocaine-primed relapse in rats: role of glutamate in the nucleus accumbens. J Neurosci 26:8531–8536
Acknowledgement
The authors wish to thank Drs. Eco de Geus and Guido Band for their advice on the analysis of the stop-signal data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Pattij, T., Janssen, M.C.W., Schepers, I. et al. Effects of the cannabinoid CB1 receptor antagonist rimonabant on distinct measures of impulsive behavior in rats. Psychopharmacology 193, 85–96 (2007). https://doi.org/10.1007/s00213-007-0773-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-007-0773-4