Abstract
Rationale
In the mouse but not the rat, d-3,4-methylenedioxymethamphetamine (d-MDMA) is a dopaminergic neurotoxicant. Various stressors and hypothermia protect against d-MDMA-induced neurotoxicity through unknown mechanisms, one of which could be a reduction in the distribution of d-MDMA to the brain.
Objectives
We determined striatal levels of d-MDMA in relation to body temperature in mice exposed to a neurotoxic regimen of d-MDMA in the presence or absence of various stressors.
Methods
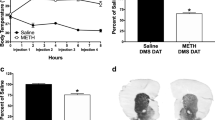
Female C57BL6/J mice received a neurotoxic regimen of d-MDMA (15.0 mg/kg s.c. as the base every 2 h×4) alone or in combination with manipulations with a known neuroprotective status. d-MDMA levels were determined by HPLC with fluorometric detection while rectal temperature provided core temperature status. Levels of dopamine, tyrosine hydroxylase and GFAP were used to assess neurotoxicity.
Results
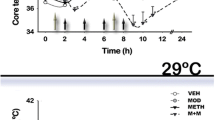
Restraint, ethanol co-treatment and cold stress were neuroprotective, caused hypothermia and increased striatal d-MDMA levels by 4- to 7-fold. Corticosterone treatment, as a stress mimic, did not alter striatal d-MDMA or temperature and was not protective. The protective glutamate receptor antagonist, MK-801, doubled striatal d-MDMA levels and caused hypothermia.
Conclusions
Although stress and other protective manipulations can alter the striatal concentration of d-MDMA their hypothermia-inducing properties appear a more likely determinant of their neuroprotection against the striatal dopaminergic neurotoxicity of d-MDMA.







Similar content being viewed by others
References
Anakk S, Kalsotra A, Shen Q, Vu MT, Staudinger JL, Davies PJ, Strol HW (2003) Genomic characterization and regulation of Cyp3a13: role of xenobiotics and nuclear receptors. FASEB J 17:1736–1738
Blume N, Leonard J, Xu ZJ, Watanabe O, Remotti H, Fishman J (2000) Characterization of Cyp2d22, a novel cytochromes P450 expressed in mouse mammary cells. Arch Biochem Biophys 381:191–204
Camarero J, Sanchez V, O’Shea E, Green AR, Colado MI (2002) Studies, using in vivo microdialysis, on the effect of the dopamine uptake inhibitor GBR12909 on 3,4-methylenedioxymethamphetamine (“ecstasy”)-induced dopamine release and free radical formation in the mouse striatum. J Neurochem 81:961–972
Carson EJ, Pruett SB (1996) Development and characterization of a binge drinking model in mice for evaluation of the immunological effects of ethanol. Alcohol Clin Exp Res 20:132–138
Chiba K, Trevor A, Castagnoli N JR (1984) Metabolism of the neurotoxic tertiary amine, MPTP, by brain monoamine oxidase. Biochem Biophys Res Commun 120:574–578
Cho AK, Hiramatsu M, Distefano EW, Chang AS, Jenden DJ (1990) Stereochemical differences in the metabolism of 3,4-methylenedioxymethamphetamine in vivo and in vitro: a pharmacokinetic analysis. Drug Metab Dispos 18:686–691
Christians U, Schmidt G, Bader A, Lampen A, Schottmann R, Linck A, Sewing KF (1996), Identification of drugs inhibiting the in vitro metabolism of tacrolimus by human liver microsomes. Br J Clin Pharmacol 41:187–190
Clausing P, Gough B, Holson RR, Slikker W Jr., Bowyer JF, (1995) Amphetamine levels in brain microdialysate, caudate/putamen, substantia nigra and plasma after dosage that produces either behavioral or neurotoxic effects. J Pharmacol Exp Ther 274:614–621
Colado MI, Camarero J, Mechan AO, Sanchez V, Estaban B, Elliot JM, Green AR (2001) A study of the mechanisms involved in the neurotoxic action of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) on dopamine neurons in mouse brain. Br J Pharmacol 134:1711–1723
Dafters RI, Lynch E (1998) Persistent loss of thermoregulation in the rat induced by 3,4-methylenedioxymethamphetamine (MDMA or “ecstasy”) but not by fenfluramine. Psychopharmacology 138:207–212
Esteban B, O’Shea E, Camarero J, Sanchez V, Green AR, Colado MI (2001) 3,4-Methylenedioxymethamphetamine induces monoamine release, but not toxicity, when administered centrally at a concentration occurring following a peripherally injected neurotoxic dose. Psychopharmacology 154:251–260
Gordon C (1993) Temperature regulation in laboratory rodents. Cambridge University Press, New York
Iber H, Chen Q, Sewer M, Morgan ET (1997) Regulation of hepatic cytochrome P450 2C11 by glucocorticoids. Arch Biochem Biophys 345:305–310
Johnson EA, Miller DB (2001) Restraint and other stress manipulations are neuroprotective against the effects of 3,4-methylenedioxymethamphetamine (d-MDMA) in C57Bl/6 J mouse because of altered distribution. Soc Neuroscience Abstr vol 27, Program no. 175.15
Johnson EA, Sharp DS, Miller DB (2000) Restraint as a stressor in mice: against the dopaminergic neurotoxicity of d-MDMA, low body weight mitigates restraint-induced hypothermia and consequent neuroprotection. Brain Res 875:107–118
Johnson EA, O’Callaghan JP, Miller DB (2002) Chronic treatment with supraphysiological levels of corticosterone enhances D-MDMA-induced dopaminergic neurotoxicity in the C57Bl/6J female mouse. Brain Res 933:130–138
Kreth KP, Kovar KA, Schwab M, Zanger UM, (2000) Identification of the human cytochromes P450 Involved in the oxidative metabolism of “ecstasy”-related designer drugs. Biochem Pharmacol 59:1563–1571
Lampen A, Christians U, Guengerich FP, Watkins PB, Kolars JC, Bader A, Gonschior AK, Dralle H, Hackbarth I, Sewing KF, (1995) Metabolism of the immunosuppressant tacrolimus in the small intestine: cytochrome P450, drug interactions, and interindividual variability. Drug Metab Dispos 23:1315–1324
Lin LY, Di Stefano EW, Schmitz DA, Hsu L, Ellis SW, Lennard MS, Tucker GT, Cho AK, (1997) Oxidation of methamphetamine and methylendioxymethamphetamine by CYP2D6. Drug Metab Dispos 25:1059–1064
Markey SP, Johannessen JN, Chiueh CC, Burns RS, Herkenham MA (1984) Intraneuronal generation of a pyridinium metabolite may cause drug-induced parkinsonism in primates. Nature 311:464–467
Maurer HH, Bickeboeller-Friedrich J, Kraemer T, Peters FT, (2000) Toxicokinetics and analytical toxicology of amphetamine-derived designer drugs (ecstasy). Toxicol Lett 112–113:133–142
Miller DB, O’Callaghan JP (1994) Environment-, drug- and stress-induced alterations in body temperature affect the neurotoxicity of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther 270:752–760
Miller DB, O’Callaghan JP (1995) The role of temperature, stress, and other factors in the neurotoxicity of the substituted amphetamines 3,4-methylenedioxymethamphetamine and fenfluramine. Mol Neurobiol 11:177–192
Molliver ME, O’Hearn E, Battaglia G, DeSouza ER (1986) Direct intracerebral administration of MDMA and MDA does not produce serotonin neurotoxicity. Soc Neurosci Abstr 12:1234
Nixdorf WL, Burrows KB, Gudelsky GA, Yamamoto BK (2001) Enhancement of 3/4-methylenedioxymethamphetamine neurotoxicity by the energy inhibitor amalonate. J Neurochem 77:647–654
O’Callaghan JP (2002) Measurement of glial fibrillary acidic protein. In: Costa LG (ed) Current protocols in toxicology. Wiley, New York, pp 12.81.1–12.8.12
O’Callaghan JP, Miller DB (1994) Neurotoxicity profiles of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther 270:741–751
O’Callaghan JP, Miller DB (2002) Neurotoxic effects of Substituted amphetamines in mice and rats: challenges to the current doga. In: Massaro EJ (ed) Handbook of neurotoxicology vol II. Humana Press, Totawa, N.J., pp 269–301
O’Loinsigh ED, Boland G, Kelly JP, O’Boyle KM (2001) Behavioral, hyperthermic and neurotoxic effects of 3,4-methylenedioxymethamphetamine analogues in the Wistar rat. Prog Neurpsychopharmacol Biol Psychiatry 25:621–638
O’Shea E, Esteban B, Camarero J, Green AR, Colado, MI (2001) Effect of GBR 12909 and fluoxetine on the acute and long term changes induced by MDMA (“ecstasy”) on the 5-HT and dopamine concentrations in mouse brain. Neuropharmacology 40:65–74
Paris JM, Cunningham KA (1992) Lack of serotonin neurotoxicity after intraraphe microinjection of (+)-3,4-methylenedioxymethamphetamine (MDMA). Brain Res Bull 28:115–119
Pruett SB, Collier SD, Wu WJ (1998) Ethanol-induced activation of the hypothalamic-pituitary-adrenal axis in a mouse model for binge drinking: role of R015-4513-sensitive gamma aminobutyric acid receptors, tolerance and relevance to humans. Life Sci 63:1137–1146
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner RH, Provenzano MD, Fujimoto EK, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Tallarida RJ, Murray RB (1987) Manual of pharmacologic calculations with computer programs, Springer-Verlag, New York, pp 77–81
Tucker GT, Lennard MS, Ellis SW, Woods HF, Cho AK, Lin LY, Hiratsuka A, Schmitz DA, Chu TYY (1994) The demethylenation of methylenedioxymethamphetamine (“ecstasy”) by debrisoquine hydroxylase (CYP2D6), Biochem Pharmacol 47:1151–1156
Wu D, Otton SV, Inaba T, Kalow W, Sellers EM (1997) Interactions of amphetamine analogs with human liver CYP2D6. Biochem Pharmacol 53:1605–1612
Acknowledgments
The authors gratefully acknowledge Fang X. Ma, Mary Ann Hammer, Bryan Wakefield, Brenda Mutnansky, and Christopher Felton for their expert technical assistance. This work was supported by intramural funds, National Institute for Occupational Safety and Health, Health Effects Laboratory Division, Toxicology and Molecular Biology Branch.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Johnson, E.A., O’Callaghan, J.P. & Miller, D.B. Brain concentrations of d-MDMA are increased after stress. Psychopharmacology 173, 278–286 (2004). https://doi.org/10.1007/s00213-003-1740-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-003-1740-3