Abstract
Rationale
Drug exposure during CNS development may alter subsequent dependence liability. We postulated that early alcohol exposure might produce persistent alterations in responses to noxious stimuli. Because relief of physical discomfort may be negatively reinforcing, changes in responses to noxious stimuli produced by early alcohol exposure may increase the rewarding properties of nicotine, a potent analgesic. Such factors may contribute to the high level of alcohol and nicotine co-abuse in humans.
Objectives
The purpose of this study was to determine whether neonatal ethanol exposure in rats altered responses to noxious stimuli, and whether nicotine would then be more rewarding to the alcohol-exposed offspring, perhaps via its analgesic actions.
Methods
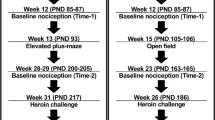
Neonatal rats received ethanol by gavage (5.0 or 6.5 g/kg) on postnatal days (PND) 9–13. An iso-caloric control group was also included. Rats were then tested to assess responsiveness to a mild noxious heat stimulus, as measured in the tail-flick assay (PND 14 and PND 28), for their response to acute analgesic injections of either nicotine or ethanol (PND 28), and for nicotine induced conditioned place preference (CPP) (PND 36).
Results
Neonatal ethanol exposure produced hyperalgesia during the first 24 h after alcohol withdrawal (PND 14) that continued through PND 28. The analgesic effects of 12.5 μg/kg nicotine were enhanced approximately 2-fold in adolescent rats with previous ethanol histories, relative to controls. These ethanol-exposed rats also showed a significant CPP to nicotine, whereas controls showed no CPP.
Conclusions
Persistent decreases in tail-flick response latencies suggestive of hyperalgesia were observed following neonatal ethanol exposure in the rat. These changes were accompanied by increases in the analgesic and place-conditioning effects of nicotine in adolescence. If similar effects occur in humans, prenatal alcohol exposure may play a role in an increased risk for the rewarding effects and dependence liability of nicotine later in life.



Similar content being viewed by others
References
Anand KJ, Scalzo FM (2000) Can adverse neonatal experiences alter brain development and subsequent behavior? Biol Neonate 77:69–82
Anand KJ, Coskun V, Thrivikraman KV, Nemeroff CB, Plotsky PM (1999) Long-term behavioral effects of repetitive pain in neonatal rat pups. Physiol Behav 66:627–637
Bienkowski P, Kuca P, Kostowski W (1995) Conditioned place preference after prolonged pre-exposure to ethanol. Pol J Pharmacol 47:189–191
Cappell MS, Schein JR (2000) Diagnosis and treatment of nonsteroidal anti-inflammatory drug-associated upper gastrointestinal toxicity. Gastroenterol Clin N Am 29:97–124
Carstens E, Anderson KA, Simons CT, Carstens MI, Jinks SL (2001) Analgesia induced by chronic nicotine infusion in rats: differences by gender and pain test. Psychopharmacology 157:40–45
Clarke PB, Fibiger HC (1987) Apparent absence of nicotine-induced conditioned place preference in rats. Psychopharmacology 92:84–88
Craft RM, Milholland RB (1998) Sex differences in cocaine- and nicotine-induced antinociception in the rat. Brain Res 809:137–140
Decker MW, McGaugh JL (1991) The role of interactions between the cholinergic system and other neuromodulatory systems in learning and memory. Synapse 7:151–168
Difranza JR, Guerrera P (1990) Alcoholism and smoking. J Stud Alcohol 51:130–135
Dobbings J, Sands J (1979) Comparative aspects of the brain growth spurt. Early Hum Dev 3:79–83
Dougherty J, Miller D, Todd G, Kostenbauder HB (1981) Reinforcing and other behavioral effects of nicotine. Neurosci Biobehav Rev 5:487–495
Faraday MM, Elliott BM, Grunberg NE (2001) Adult vs adolescent rats differ in biobehavioral responses to chronic nicotine administration. Pharmacol Biochem Behav 70:475–489
Fillingim RB, Ness TJ (2000) Sex-related hormonal influences on pain and analgesic responses. Neurosci Biobehav Rev 24:485–501
Fitzgerald M, Millard C, McIntosh N (1988) Hyperalgesia in premature infants. Lancet 8580:292
Franklin KB (1998) Analgesia and abuse potential: An accidental association or a common substrate? Pharmacol Biochem Behav 59:993–1002
Fudala PJ, Teoh KW, Iwamoto ET (1985) Pharmacologic characterization of nicotine-induced conditioned place preference. Pharmacol Biochem Behav 22:237–241
Gabriel KI, Yu W, Ellis L, Weinberg J (2000) Postnatal handling does not attenuate hypothalamic-pituitary-adrenal hyperresponsiveness after prenatal ethanol exposure. Alcohol Clin Exp Res 24:1566–1574
Gatch MB, Lal H (1999) Effects of ethanol and ethanol withdrawal on nociception in rats. Alcohol Clin Exp Res 23:328–333
Gear RW, Levine JD (1995) Antinociception produced by an ascending spino-supraspinal pathway. J Neurosci 15:3154–3161
Gear RW, Aley KO, Levine JD (1999) Pain-induced analgesia mediated by mesolimbic reward circuits. J Neurosci 19:7175–7181
Goodlett CR, Johnson TB (1997) Neonatal binge ethanol exposure using intubation: timing and dose effects on place learning. Neurotoxicol Teratol 19:435–446
Herrero JF, Laird J, Lopez-Garcia JA (2000) Windup of spinal neurones and pain sensation: much ado about something? Prog Neurobiol 61:169–203
Hildebrand BE, Nornikos GG, Hertel P, Schilstrom B, Svensson TH (1997) Behavioral manifestations of the nicotine abstinence syndrome in the rat: peripheral versus central mechanisms. Psychopharmacology 129:348–356
Hirakawa N, Tershner SA, Fields HL, Manning BH (2000) Bi-directional changes in affective state elicited by manipulation of medullary pain control circuitry. Neuroscience 100: 861–871
Horan B, Smith M, Gardner EL, Lepore M, Ashby CR Jr (1997) (−)-Nicotine produces conditioned place preference in Lewis, but not Fischer 344 rats. Synapse 26:93–94
Hu XJ, Ticku MK (1995) Chronic ethanol treatment up-regulates the NMDA receptor function and binding in mammalian cortical neurons. Mol Brain Res 30:347–356
Hughes JR, Rose GL, Callas PW (2000) Nicotine is more reinforcing in smokers with a past history of alcoholism than in smokers without this history. Alcohol Clin Exp Res 24:1633–1638
Iwamoto ET (1989) Antinociception after nicotine administration in the mesopontine tegmentum of rats: evidence for muscarinic actions. J Pharmacol Exp Ther 251:412–421
Iwamoto ET (1991) Characterization of the antinociception induced by nicotine in the pedunculopontine tegmental nucleus and the nucleus raphe magnus. J Phamacol Exp Ther 257:120–133
Iwamoto ET, Martin W (1988) A critique of drug self-administration as a method for predicting abuse potential of drugs. NIDA Res Monogr 1046:81457–81465
Jamner LD, Girdler SS, Shapiro D, Jarvik ME (1998) Pain inhibition, nicotine and gender. Exp Clin Psychopharmacol 6:96–106
Kandel DB, Chen K (2000) Extent of smoking and nicotine dependence in the United States: 1991–1993. Nicotine Tob Res 2:263–274
Kandel DB, Yamaguchi K (1985) Developmental patterns of the use of legal, illegal, and medically prescribed psychotropic drugs from adolescence to young adulthood. In: Etiology of drug abuse: implications for prevention. NIDA Res Monogr 56:193–235
Kentroti S, Vernadakis A (1996) Ethanol neurotoxicity in culture: selective loss of cholinergic neurons. J Neurosci Res 44:577–585
Koob GF, LeMoal M (1997) Drug abuse: hedonic homeostatic dysregulation. Science 278:52–58
Koob GF, LeMoal M (2001) Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24:97–129
Lester BM, Tronick EZ, LaGasse L, Seifer R, Bauer CR, Shankaran S, Bada HS, Wright LL, Smeriglio VL, Lu J, Finnegan LP, Maza PL (2002) The maternal lifestyle study: effects of substance exposure during pregnancy on neurodevelopmental outcome in 1-month-old infants. Pediatrics 110:1182–1192
Li X, Eisenach JC (2002) Nicotinic acetylcholine receptor regulation of spinal norepinephrine release. Anesthesiology 96:1450–1456
Littleton JM, Lovinger D, Liljequist S, Ticku R, Matsumoto I, Barron S (2001) Role of polyamines and NMDA receptors in ethanol dependence and withdrawal. Alcohol Clin Exp Res 25:132S–136S
Malin DH, Lake JR, Newlin-Maultsby P, Roberts LK, Lanier JG, Carter VA, Cunningham JS, Wilson OB (1992) Rodent model of nicotine abstinence syndrome. Pharmacol Biochem Behav 43: 779–784
Matsuzawa S, Suzuki T, Misawa M (2000) Ethanol, but not the anxiolytic drugs buspirone and diazepam, produces a conditioned place preference in rats exposed to conditioned fear stress. Pharmacol Biochem Behav 65:281–288
Nagahara AH, Handa RJ (1999a) Fetal alcohol-exposed rats exhibit differential response to cholinergic drugs on a delay-dependent memory task. Neurobiol Learn Mem 72:230–243
Nagahara AH, Handa RJ (1999b) Loss of nicotine-induced effects on locomotor activity in fetal alcohol-exposed rats. Neurotoxicol Teratol 21:647–652
Nagy J, Muller F, Laszlo L (2001) Cytotoxic effect of alcohol withdrawal on primary cultures of cortical neurones. Drug Alcohol Depend 61:155–162
Nelson DE, Giovino GA, Shopland DR, Mowery PD, Mills SL, Eriksen MP (1995) Trends in cigarette smoking among US adolescents, 1974 thru 1991. Am J Public Health 85 34–40
Nelson LR, Taylor AN, Lewis JW, Branch BJ, Liebeskind JC (1986) Morphine analgesia is potentiated in adult rats prenatally exposed to ethanol. Brain Res 372:234–240
Panagis G, Nisell M, Nomikos GG, Chergui K, Svensson TH (1996) Nicotine injections into the ventral tegmental area increase locomotion and Fos-like immunoreactivity in the nucleus accumbens of the rat. Brain Res 730:133–142
Patten CA, Martin JE, Owen N (1996) Can psychiatric and chemical dependency treatment units be smoke free? J Subst Abuse Treat 13:107–118
Plenge P, Mellerup ET, Wortwein G (2002) Characterization of epibatidine binding to medial habenula: potential role in analgesia. J Pharmacol Exp Ther 302:759–765
Prescott J (2000) Paracetamol, alcohol and the liver. Br J Clin Pharmacol 49:291–301
Rao TS, Correa LD, Reid RT, Lloyd K (1996) Evaluation of antinociceptive effects of neuronal acetylcholine receptor ligands in the rat tail-flick assay. Neuropharmacology 35:393–405
Reid LD, Hunter GA, Beaman CM, Hubbell CL (1985) Toward understanding ethanol's capacity to be reinforcing: a conditioned place preference following injections of ethanol. Pharmacol Biochem Behav 22:483–487
Rogers DT, Iwamoto ET (1993) Multiple spinal mediators in parenteral nicotine-induced antinociception. J Pharmacol Exp Ther 261:91–96
Ruda MA, Ling Q, Hohmannn P, Peng YB, Tachibana T (2000) Altered nociceptive neuronal circuits after neonatal peripheral inflammation. Science 289:628–630
Rudeen PK, Weinberg J (1993) Prenatal ethanol exposure: changes in regional brain catecholamine content following stress. J Neurochem 61:1907–1915
Samson HH, Czachowski CL, Slawecki CJ (2000) A new assessment of the ability of oral ethanol to function as a reinforcing stimulus. Alcohol Clin Exp Res 24:766–773
Schmidt BL, Tambell CH, Gear RW, Levine JD (2001) Nicotine withdrawal hyperalgesia and opioid-mediated analgesia depend on nicotine receptors in the nucleus accumbens. Neuroscience 106:129–136
Shoaib M, Stolerman IP, Kumar RC (1994) Nicotine-induced place preferences following prior nicotine exposure in rats. Psychopharmacology 113:445–452
Thomas JD, Wasserman EA, West JR, Goodlett CR (1996) Behavioral deficits induced by binge-like exposure to alcohol in neonatal rats: importance of developmental timing and number of episodes. Dev Psychobiol 29:433–452
Thomas JD, La Fiette MH, Quinn VR, Riley EP (2000) Neonatal choline supplementation ameliorates the effects of prenatal alcohol exposure on a discrimination learning task in rats. Neurotoxicol Teratol 22:703–711
Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP (2002) Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav 77:107–114
Webber RA, Hunter M, Keats AJ (1994) Peer and parental influences on adolescents' substance use: a path analysis. Int J Addict 29:647–657
Weinberg J (1992) Prenatal ethanol exposure alters adrenocortical responses to predictable and unpredictable stressors. Alcohol 9:427–432
Weinberg J, Taylor AW, Gianoulakis C (1996) Fetal ethanol exposure: hypothalamic-pituitary-adrenal and beta-endorphin responses to repeated stress. Alcohol Clin Exp Res 20:122–131
West JR, Hamre KM, Pierce DR (1984) Delay in brain growth induced by alcohol in artificially-reared rat pups. Alcohol 1:213–222
West JR, Goodlett CR, Bonthius DJ, Hamre KM, Marcussen BL (1990) Cell population depletion associated with fetal alcohol brain damage: mechanisms of BAC-dependent cell loss. Alcohol Clin Exp Res 14:813–818
Yates WR, Cadoret RJ, Troughton EP, Stewart M, Giunta T (1998) Effect of fetal alcohol exposure on adult symptoms of nicotine, alcohol, and drug dependence. Alcohol Clin Exp Res 22:914–920
Zbuzek VK, Chin WY (1994) Prenatal nicotine exposure increased duration of nicotine-induced analgesia in adult rats. Psychopharmacology 113:534–538
Acknowledgements
The authors wish to thank Dr. Michael Bardo for his assistance in the design and execution of this study and Dr. Kurt Hauser for loan of equipment. We also thank the Kentucky Tobacco Research and Development Center (KTRDC) for its continued support. KTRDC is an administrative unit of the University of Kentucky, and is not associated with the tobacco industry.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rogers, D.T., Barron, S. & Littleton, J.M. Neonatal ethanol exposure produces a hyperalgesia that extends into adolescence, and is associated with increased analgesic and rewarding properties of nicotine in rats. Psychopharmacology 171, 204–211 (2004). https://doi.org/10.1007/s00213-003-1574-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-003-1574-z