Abstract
Rationale
Previously, we have shown that d-amphetamine (AMPH) was more potent than d-methamphetamine (METH) at increasing extracellular levels of dopamine (DA) in the prefrontal cortex (PFC) at doses that had similar effects in the nucleus accumbens. Since working memory depends on PFC DA, it was postulated that AMPH would also be more potent than METH at affecting working memory.
Objective
To determine if AMPH is more potent than METH at affecting working memory.
Methods
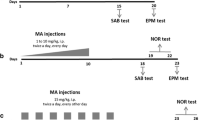
Working memory was measured in adult female Sprague-Dawley rats using a delayed-alternation T-maze task with multiple delays (1, 10, 60 s) and food rewards. The percentage of food rewards consumed was also recorded. Animals were tested with METH and AMPH before and after a chronic protocol, with measurements of locomotor activity used to test for pharmacological tolerance or sensitization. The effects of METH and AMPH on extinction were also examined by omitting the food rewards from the T-maze.
Results
Both METH and AMPH produced dose-related bimodal effects on working memory at the intermediate delay (10 s); however, AMPH was more potent than METH. Both METH and AMPH initially also decreased the percentage of food rewards consumed in the T-maze. After chronic testing, animals displayed tolerance to both the working memory impairments and the reduction in food reward intake produced by AMPH. Animals did not display significant tolerance to the effects of METH on food reward consumption and performed worse in the T-maze after chronic testing. METH, but not AMPH, interfered with extinction.
Conclusions
These results indicate that METH and AMPH differ in altering working memory and the expression of tolerance, perhaps due to differences in behavioral inhibition.






Similar content being viewed by others
References
Aultman JM, Moghaddam B (2001) Distinct contributions of glutamate and dopamine receptors to temporal aspects of rodent working memory using a clinically relevant task. Psychopharmacology 153:353–364
Cummings JL (1995) Anatomic and behavioral aspects of frontal-subcortical circuits. Ann NY Acad Sci 769:1–13
Dehaene S, Changeux JP (2000) Reward-dependent learning in neuronal networks for planning and decision making. Prog Brain Res 126:217–229
Glick SD, Jerussi TP, Zimmerberg B (1977) Behavioral and neuropharmacological correlates of nigro-striatal asymmetry in rats. In: Harnad S (ed) Lateralization in the nervous system. Academic Press, New York, pp 213–249
Goldman-Rakic PS (1995) Cellular basis of working memory. Neuron 14:477–485
Goldman-Rakic PS, Muly EC, Williams GV (2000) D(1) receptors in prefrontal cells and circuits. Brain Res Brain Res Rev 31:295–301
Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A (1996) Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA 93:12040–12045
Hill RT (1969) Facilitation of conditioned reinforcement as a mechanism of psychomotor stimulation. In: Costa E, Garattini S (eds) International symposium on amphetamine and related compounds. Raven Press, New York, pp 781–795
Jentsch JD, Taylor JR (1999) Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology 146:373–390
Kandel D, Doyle D, Fischman MW (1975) Tolerance and cross-tolerance to the effects of amphetamine, methamphetamine and fenfluramine on milk consumption in the rat. Pharmacol Biochem Behav 3:705–707
Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, Kukes TJ, Renshaw PF (1998) Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry 155:124–126
McFarland K, Kalivas PW (2001) The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 21:8655–8663
Park WK, Bari AA, Jey AR, Anderson SM, Spealman RD, Rowlett JK, Pierce RC (2002) Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J Neurosci 22:2916–2925
Schuster CR, Zimmerman J (1961) Timing behavior during prolonged treatment with dl-amphetamine. J Exp Anal Behav 4:327–330
Schuster CR, Dockens WS, Woods JH (1966) Behavioral variables affecting the development of amphetamine tolerance. Psychopharmacologia 9:170–182
Self DW (1998) Neural substrates of drug craving and relapse in drug addiction. Ann Med 30:379–389
Shoblock JR, Sullivan EB, Maisonneuve IM, Glick SD (2003) Neurochemical and behavioral differences between d-methamphetamine and d-amphetamine. Psychopharmacology 165:359–369
Taylor JR, Robbins TW (1984) Enhanced behavioural control by conditioned reinforcers following microinjections of d-amphetamine into the nucleus accumbens. Psychopharmacology 84:405–412
Tzschentke TM (2001) Pharmacology and behavioral pharmacology of the mesocortical dopamine system. Prog Neurobiol 63:241–320
Weinberger DR (1993) A connectionist approach to the prefrontal cortex. J Neuropsychiatr Clin Neurosci 5:241–253
Weissenborn R, Robbins TW, Everitt BJ (1997) Effects of medial prefrontal or anterior cingulate cortex lesions on responding for cocaine under fixed-ratio and second-order schedules of reinforcement in rats. Psychopharmacology 134:242–257
Wolgin DL (2000) Contingent tolerance to amphetamine hypophagia: new insights into the role of environmental context in the expression of stereotypy. Neurosci Biobehav Rev 24:279–294
Wolgin DL, Hughes KM (1996) Effect of sensitization of stereotypy on the acquisition and retention of tolerance to amphetamine hypophagia. Psychopharmacology 126:219–225
Wolgin DL, Wade JV (1995) Learned suppression of stereotypy in amphetamine-treated rats: implications for understanding tolerance to amphetamine "anorexia". Behav Pharmacol 6:254–262
Zimmerman J, Schuster CR (1962) Spaced responding in multiple DRL schedules. J Exp Anal Behav 5:497–504
Acknowledgements
This study was supported by NIDA grants DA03817 and DA07307.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shoblock, J.R., Maisonneuve, I.M. & Glick, S.D. Differences between d-methamphetamine and d-amphetamine in rats: working memory, tolerance, and extinction. Psychopharmacology 170, 150–156 (2003). https://doi.org/10.1007/s00213-003-1522-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-003-1522-y