Abstract
Rationale
Neonatal administration of methamphetamine (MA) to rats from postnatal day (P) 11 to 20, but not from P1 to P10, produces lasting deficits in spatial learning and memory. The preweaning period of development in the rat corresponds to human third trimester hippocampal development and because of the increased use of MA in women of childbearing age, there is a greater likelihood that fetuses will be exposed to this drug. Development of the hippocampus is dependent upon many factors, including an optimal level of corticosterone (CORT). We have demonstrated that the CORT response of animals on P11 to MA is protracted relative to administration on P15 or P20. Interestingly, the P11 animals are still in the stress hyporesponsive period.
Objectives
We postulated that because of the prolonged CORT response on P11, the effects of MA on spatial learning and memory may be confined to a shorter period of exposure.
Methods
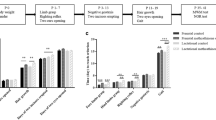
Neonatal rats were administered MA (10 mg/kg) 4 times daily from either P11 to P15 or from P16 to P20, raised to adulthood and tested against animals only administered saline (SAL) from P11 to P20 for anxiety, swimming ability, and spatial learning and memory.
Results
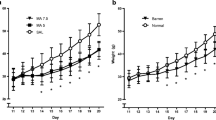
Animals exposed to MA, regardless of exposure period, tended to be less anxious in the Zero maze relative to SAL animals. No differences were noted for swimming ability. Only animals exposed to MA from P11 to P15 demonstrated deficits in spatial learning and memory during acquisition as well as during a shifted platform phase where learning a new position was required.
Conclusions
The results demonstrate that spatial learning and memory deficits produced by MA administration are dependent upon when the exposure of the animal occurs and appears to be during the period of development in the rat when the response to threatening environments, stressors, is greatly reduced.



Similar content being viewed by others
References
Almli CR, Levy TJ, Han BH, Shah AR, Gidday JM, Holtzman DM (2000) BDNF protects against spatial memory deficits following neonatal hypoxia-ischemia. Exp Neurol 166:99–114
Bayer SA, Altman J, Russo RJ, Zhang X (1993) Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology 14:83–144
Behan DP, Heinrichs SC, Troncoso JC, Liu X-J, Kawas CH, Ling N, De Souza EB (1995) Displacement of corticotropin releasing factor from its binding protein as a possible treatment for Alzheimer's disease. Nature 378:284–287
Blass EM, Teicher MH (1980) Suckling. Science 210:15–22
Broening HW, Morford LL, Inman-Wood SL, Fukumura M, Vorhees CV (2001) 3,4-methylenedioxymethamphetamine (Ecstasy)-induced learning and memory impairments depend on the age of exposure during early development. J Neurosci 21:3228–3235
Cappon G, Morford LL, Vorhees CV (1997) Ontogeny of methamphetamine-induced neurotoxicity and associated hyperthermic response. Dev Brain Res 103:155–162
Cernerud L, Eriksson M, Jonsson B, Steneroth G, Zetterström R (1996) Amphetamine addiction during pregnancy: 14-year follow-up of growth and school performance. Acta Paediatr 85:204–208
Chen Y, Bender RA, Frotscher M, Baram TZ (2001) Novel and transient populations of corticotropin-releasing hormone-expressing neurons in developing hippocampus suggest unique functional roles: a quantitative spatiotemporal analysis. J Neurosci 21:7171–7181
Conklin P, Heggeness FW (1971) Maturation of temperature homeostasis in the rat. Am J Physiol 220:333–336
Conrad CD, Roy EJ (1995) Dentate gyrus destruction and spatial learning impairment after corticosteroid removal in young and middle-aged rats. Hippocampus 5:1–15
Conrad CD, Lupien SJ, McEwen BS (1999) Support for a bimodal role for Type II adrenal steroid receptors in spatial memory. Neurobiol Learn Mem 72:39–46
Contet C, Rawlins JNP, Bannerman DM (2001) Faster is not surer—a comparison of C57BL/6J and 129S2/Sv mouse strains in the watermaze. Behav Brain Res 125:261–267
Das KP, Chao SL, White LD, Haines WT, Harry GJ, Tilson HA, Barone S Jr (2001) Differential patterns of nerve growth factor, brain-derived neurotrophic factor and neurotrophin-3 mRNA and protein levels in developing regions of rat brain. Neuroscience 103:739–761
Dixon SD, Bejar R (1989) Echoencephalographic findings in neonates associated with maternal cocaine and methamphetamine use: incidence and clinical correlates. J Pediatr 115:770–778
Farfel GM, Seiden LS (1995) Role of hypothermia in the mechanism of protection against serotonergic toxicity. II. Experiments with methamphetamine, p-chloroamphetamine, fenfluramine, dizocilpine and dextromethorphan. J Pharmacol Exp Ther 272:868–875
Frost DO, Cadet JL (2000) Effects of methamphetamine-induced neurotoxicity on the development of neural circuitry: a hypothesis. Brain Res Rev 34:103–118
Gallagher M, Burwell R, Burchinal M (1993) Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci 107:618–626
Gould E, Woolley CS, Cameron HA, Daniels DC, McEwen BS (1991a) Adrenal steroids regulate postnatal development of the rat dentate gyrus: II. Effects of glucocorticoids and mineralocorticoids on cell birth. J Comp Neurol 313:486–493
Gould E, Woolley CS, McEwen BS (1991b) Adrenal steroids regulate postnatal development of the rat dentate gyrus: I. Effects of glucocorticoids on cell death. J Comp Neurol 313:479–485
Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG (2002) Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long-Evans rats. Pharmacol Biochem Behav 73:131–140
Leakey JE, Fouts JR (1979) Precocious development of cytochrome P-450 in neonatal rat liver after glucocorticoid treatment. Biochem J 182:233–235
Lindner MD (1997) Reliability, distribution, and validity of age-related cognitive deficits in the Morris water maze. Neurobiol Learn Mem 68:203–220
Little BB, Snell LM, Gilstrap III LC (1988) Methamphetamine abuse during pregnancy: outcome and fetal effects. Obstet Gynecol 72:541–544
Maurer R, Derivaz V (2000) Rats in a transparent morris water maze use elemental and configural geometry of landmarks as well as distance to the pool wall. Spatial Cognit Computat 2:135–156
Meaney MJ, Sapolsky RM, McEwen BS (1985) The development of the glucocorticoid receptor system in the rat limbic brain. I. Ontogeny and autoregulation. Dev Brain Res 18:159–164
Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T (2000) Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J Neurosci 20:7116–7121
Morimasa T, Wirz-Justice A, Kraeuchi K, Arendt J, Baumann J, Haeusler A, Degen P, Feer H (1987) Chronic methamphetamine and its withdrawl modify behavioral and neuroendocrine circadian rhythms. Physiol Behav 39:699–705
Morris RGM (1981) Spatial localization does not require the presence of local cues. Learn Motivat 12:239–260
Morris RGM, Garrud P, Rawlins JNP, O'Keefe J (1982) Place navigation impaired in rats with hippocampal lesions. Nature 297:681–683
National Institute on Drug Abuse Research Report (1998) Methamphetamine: abuse and addiction. NIH Publication No 98-4210, 1–3
O'Callaghan JP, Miller DB (2000) Neurotoxic effects of substituted amphetamines in mice and rats: challenges to the current dogma. In: Massaro E, Broderick PA (eds) Handbook of Neurotoxicity. Humana Press, New York
O'Dell SJ, Marshall JF (2002) Effects of vibrissae removal on methamphetamine-induced damage to rat somatosensory cortical neurons. Synapse 43:122–130
Oro AS, Dixon SD (1987) Perinatal cocaine and methamphetamine exposure: maternal and neonatal correlates. J Pediatr 111:571–578
Pu C, Vorhees CV (1993) Developmental dissociation of methamphetamine-induced depletion of dopaminergic terminals and astrocyte reaction in rat striatum. Dev Brain Res 72:325–328
Redman RS, Sweney LR (1976) Changes in diet and patterns of feeding activity in developing rats. J Nutr 106:615–626
Rice D, Barone S Jr (2000) Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect 108:511–533
Sapolsky RM, Meaney MJ (1986) Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res Rev 11:65–76
Schaaf MJM, de Kloet ER, Vreugdenhil E (2000) Corticosterone efffects on BDNF expression in the hippocampus: Implications for memory formation. Stress 3:201–208
Schaaf MJM, Workel JO, Lesscher HM, Vreugdenhil E, Oitzl MS, de Kloet ER (2001) Correlation between hippocampal BDNF mRNA expression and memory performance in senescent rats. Brain Res 915:227–233
Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT (1994) Behavioural and pharmacological characterisation of the elevated "zero-maze" as an animal model of anxiety. Psychopharmacology 116:56–64
Smith LM, Chang L, Yonekura ML, Grob C, Osborn D, Ernst T (2001) Brain proton magnetic resonance spectroscopy in children exposed to methamphetamine in utero. Neurology 57:255–260
Struthers JM, Hansen RL (1992) Visual recognition memory in drug-exposed infants. J Dev Behav Pediatr 13:108–111
Vorhees CV, Ahrens KG, Acuff-Smith KD, Schilling MA, Fisher JE (1994) Methamphetamine exposure during early postnatal development in rats: I. Acoustic startle augmentaion and spatial learning deficits. Psychopharmacology 114:392–401
Vorhees CV, Reed TM, Schilling MA, Fisher JE, Moran MS, Cappon GD, Nebert DW (1998) CYP2D1 polymorphism in methamphetamine-treated rats: genetic differences in neonatal mortality and effects on spatial learning and acoustic startle. Neurotoxicol Teratol 20:265–273
Vorhees CV, Morford LL, Inman SL, Reed TM, Schilling MA, Cappon GD, Moran MS, Nebert DW (1999) Genetic differences in spatial learning between Dark Agouti and Sprague-Dawley strains: possible correlation with the CYP2D2 polymorphism in rats treated neonatally with methamphetamine. Pharmacogenetics 9:171–181
Vorhees CV, Inman-Wood SL, Morford LL, Broening HW, Fukumura M, Moran MS (2000) Adult learning deficits after neonatal exposure to d-methamphetamine: selective effects on spatial navigation and memory. J Neurosci 20:4732–4739
Williams MT, Inman-Wood SL, Morford LL, McCrea AE, Ruttle AM, Moran MS, Rock SL, Vorhees CV (2000) Preweaning treatment with methamphetamine induces increases in both corticosterone and ACTH in rats. Neurotoxicol Teratol 22:751–759
Williams MT, Vorhees CV, Boon F, Saber AJ, Cain DP (2002) Methamphetamine exposure from postnatal day 11 to 20 causes impairments in both behavioral strategies and spatial learning in adult rats. Brain Res 958:312–321
Williams MT, Morford LL, Wood SL, Wallace TL, Fukumura M, Broening HW, Vorhees CV (2003) Developmental d-methamphetamine treatment selectively induces spatial navigation impairments in reference memory in the Morris water maze while sparing working memory. Synapse 48:138–148
Wilson JG (1973) Environment and birth defects. Academic Press, New York
Acknowledgements
This study was supported by NIH research grants DA06733 (C.V.V.) and DA14269 (M.T.W.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Williams, M.T., Moran, M.S. & Vorhees, C.V. Refining the critical period for methamphetamine-induced spatial deficits in the Morris water maze. Psychopharmacology 168, 329–338 (2003). https://doi.org/10.1007/s00213-003-1433-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-003-1433-y