Abstract
Rationale
Clozapine has been shown to increase extracellular dopamine (DA) and noradrenaline (NA) in the medial prefrontal cortex (mPFC). A recent study of ours suggested that extracellular DA in the PFC originates not only from dopaminergic but also from noradrenergic terminals, its release being controlled by α2-adrenoceptors.
Objectives
Since clozapine binds to α2-adrenoceptors, the possibility that it might co-release DA and NA was studied.
Methods
By means of microdialysis coupled to HPLC with electrochemical detection, the effect of clozapine on extracellular DA and NA in the mPFC, densely innervated by DA and NA, was compared to that in the occipital cortex, equally innervated by NA but receiving few DA projections.
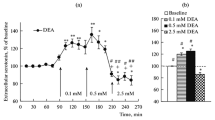
Results
Extracellular NA was found to be the same in the two cortices, consistent with homogeneous NA innervation. On the other hand, extracellular DA in the occipital cortex was only 29% lower than in the mPFC, in spite of the scarce dopaminergic innervation in the occipital cortex. Clozapine (10 mg/kg IP) increased extracellular DA and NA not only in the mPFC (by about 320% and 290%, respectively) but also in the occipital cortex (by 560% and 230%, respectively). Administration of the α2-agonist clonidine (0.15 mg/kg) reversed the effect of clozapine in both cortices, while the D2-agonist quinpirole (0.1 mg/kg IP) was ineffective.
Conclusions
The results suggest that clozapine, by inhibiting α2-adrenoceptors, co-releases DA and NA from noradrenergic terminals in the occipital cortex and that the same mechanism might be responsible for the concomitant increase of the two monoamines in the mPFC.


Similar content being viewed by others
References
Baldessarini RJ, Frankenburg FR (1991) Clozapine. A novel antipsychotic agent. N Engl J Med 324:746–754
Bymaster FP, Calligaro DO, Falcone JF, Marsh RD, Moore NA, Tye NC, Seeman P, Wong DT (1996) Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology 14:87–96
Carboni E, Tanda GL, Frau R, Di Chiara G (1990) Blockade of the noradrenaline carrier increases extracellular dopamine concentrations in the prefrontal cortex: evidence that dopamine is taken up in vivo by noradrenergic terminals. J Neurochem 55:1067–1070
Carpenter WT, Magno Zito J, Vitrai J, Volavka J (1998) Hypothesis testing: is clozapine's superior efficacy dependent on moderate D2 receptor occupancy? Biol Psychiatry 43:79–83
Casu MA, Colombo G, Gessa GL, Pani L (2002) Reduced TH-immunoreactive fibers in the limbic system of Sardinian alcohol-preferring rats. Brain Res 924:242–251
Chen JP, Ruan D, Paredes W, Gardner EL (1992) Effects of acute and chronic clozapine on dopaminergic function in medial prefrontal cortex of awake, freely moving rats. Brain Res 571:235–241
Devoto P, Flore G, Pani L, Gessa GL (2001) Evidence for co-release of noradrenaline and dopamine from noradrenergic neurons in the cerebral cortex. Mol Psychiatry 6:657–664
Gessa GL, Devoto P, Diana M, Flore G, Melis M, Pistis M (2000) Dissociation of haloperidol, clozapine and olanzapine effects on electrical activity of mesocortical dopamine neurons and dopamine release in the prefrontal cortex. Neuropsychopharmacology 22:642–649
Gresch PJ, Sved AF, Zigmond MJ, Finlay JM (1995) Local influence of endogenous norepinephrine on extracellular dopamine in rat medial prefrontal cortex. J Neurochem 65:111–116
Heidbreder CA, Foxton R, Cilia J, Hughes ZA, Shah AJ, Atkins A, Hunter AJ, Hagan JJ, Jones DNC (2001) Increased responsiveness of dopamine to atypical, but not typical antipsychotics in the medial prefrontal cortex of rats reared in isolation. Psychopharmacology 156:338–351
Hertel P, Fagerquist MV, Svensson TH (1999a) Enhanced cortical dopamine output and antipsychotic-like effects of raclopride by α2-adrenoceptor blockade. Science 286:105–107
Hertel P, Nomikos GG, Svensson TH (1999b) Idazoxan preferentially increases dopamine output in the rat medial prefrontal cortex at the nerve terminal level. Eur J Pharmacol 371:153–158
Imperato A, Angelucci L (1989) The effect of clozapine and fluperlapine on the in vivo release and metabolism of dopamine in the striatum and in the prefrontal cortex of freely moving rats. Psychopharmacol Bull 25:383–389
Imperato A, Tanda G, Frau R, Di Chiara G (1987) Pharmacological profile of dopamine receptor agonists as studied by brain dialysis in behaving rats. J Pharmacol Exp Ther 245:257–264
Kane J, Honigfeld G, Singer J, Meltzer H (1988) Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry 45:789–796
Kuroki T, Meltzer HY, Ichikawa J (1999) Effect of antipsychotic drugs on extracellular dopamine levels in rat medial prefrontal cortex and nucleus accumbens. J Pharmacol Exp Ther 288:774–781
Li X-M, Perry KW, Wong DT, Bymaster FP (1998) Olanzapine increases in vivo dopamine and noradrenaline release in rat prefrontal cortex, nucleus accumbens and striatum. Psychopharmacology 136:153–161
Lidow MS, Williams GV, Goldman-Rakic PS (1998) The cerebral cortex: a case for a common site of action of antipsychotics. Trends Pharmacol Sci 19:136–140
Lieberman J, Johns C, Cooper T, Pollack S, Kane J (1989) Clozapine pharmacology and tardive dyskinesia. Psychopharmacology 99:S54–59
Lindvall O, Björklund A, Divac I (1978) Organization of the catecholamine neurons projecting to the frontal cortex in the rat. Brain Res 142:1–24
Meltzer HY, Stahl SM (1976) The dopamine hypothesis of schizophrenia: a review. Schizophr Bull 2:19–76
Moghaddam B, Bunney BS (1990) Acute effects of typical and atypical antipsychotic drugs on the release of dopamine from prefrontal cortex, nucleus accumbens, and striatum of the rat: an in vivo microdialysis study. J Neurochem 54:1755–1760
Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT (2002) Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mice lines. J Neurosci 22:389–395
Nutt DJ, Lalies MD, Lione LA, Hudson AL (1997) Noradrenergic mechanism in the prefrontal cortex. J Psychopharmacol 11:163–168
Paxinos G, Watson C (1997) The rat brain in stereotaxic co-ordinates. Academic Press, New York
Pehek EA, Yamamoto BK (1994) Differential effects of locally administered clozapine and haloperidol on dopamine efflux in the rat prefrontal cortex and caudate-putamen. J Neurochem 63:2118–2124
Ramirez OA, Wang RY (1986) Locus coeruleus norepinephrine-containing neurons: effects produced by acute and subchronic treatment with antipsychotic drugs and amphetamine. Brain Res 362:165–170
Smiley JF, Levey AI, Ciliax BJ, Goldman-Rakic PS (1994) D1 dopamine receptor immunoreactivity in human and monkey cerebral cortex: predominant and extrasynaptic localization in dendritic spines. Proc Natl Acad Sci USA 91:5720–5724
Souto M, Monti JM, Altier H (1979) Effects of clozapine on the activity of central dopaminergic and noradrenergic neurons. Pharmacol Biochem Behav 10:5–9
Sulser F, Vetulani F, Mobley P (1978) Mode of action of antidepressant drugs. Biochem Pharmacol 27:257–261
Svensson TH, Bunney BS, Aghajanian GK (1975) Inhibition of both noradrenergic and serotonergic neurons in brain by the α-adrenergic agonist clonidine. Brain Res 92:291–306
Westerink BHC, de Boer P, de Vries JB, Kruse CG, Long SK (1998) Antipsychotic drugs induce similar effects on the release of dopamine and noradrenaline in the medial prefrontal cortex of the rat brain. Eur J Pharmacol 361:27–33
White FJ, Wang RY (1984) Pharmacological characterization of dopamine autoreceptors in the rat ventral tegmental area: microiontophoretic studies. J Pharmacol Exp Ther 231: 275–280
Williams GV, Goldman-Rakic PS (1995) Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature 376:572–575
Yamamoto BK, Novotney S (1998) Regulation of extracellular dopamine by the norepinephrine transporter. J Neurochem 71:274–280
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Devoto, P., Flore, G., Vacca, G. et al. Co-release of noradrenaline and dopamine from noradrenergic neurons in the cerebral cortex induced by clozapine, the prototype atypical antipsychotic. Psychopharmacology 167, 79–84 (2003). https://doi.org/10.1007/s00213-002-1381-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-002-1381-y