Abstract
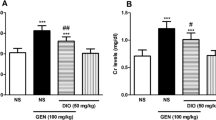
Neurotoxicity and nephrotoxicity are the major dose-limiting factors for the clinical use of colistin against multidrug-resistant (MDR) Gram-negative bacteria. This study aimed to investigate the neurotoxic and nephrotoxic effects of colistin formulated with in-house synthesized sodium deoxycholate sulfate (SDCS) in a mouse model. Male mice C57BL/6 were randomly divided into four groups: control (saline solution), colistin (15 mg/kg/day), colistin:SDCS 1:1, and colistin:SDCS 1:2. In the colistin:SDCS treatment groups, the dosage was 15 mg/kg/day colistin equivalent; all mice were treated for 7 successive days. The thermal tolerance, body weight gain and organ weights were measured. The levels of serum blood urea nitrogen (BUN), creatinine (Cr), superoxide dismutase (SOD), and catalase (CAT) were assessed. Histopathological damages were assessed on mice organ. The colistin:SDCS formulations significantly improved thermal pain response of the mice comparable to the control group. The administration did not impair kidney function as evidence from BUN and Cr results; however, the oxidative stress biomarkers decreased in the colistin and colistin-SDCS treated mice. Several abnormalities were observed in the kidney, liver, spleen, and sciatic nerve tissues following colistin treatment, which indicated evidence of toxicity. The colistin-SDCS formulations were associated with less acute toxicity and fewer nephrotoxic and neurotoxic changes compared with the colistin alone group which indicated that SDCS attenuated colistin nephrotoxicity and neurotoxicity. This study highlights the potential application of colistin formulated with SDCS for safer clinical use against MDR Gram-negative bacteria.
Graphical Abstract







Similar content being viewed by others
Data availability
The data will be made available upon request.
References
Ahmed MAEE, Doi Y, Tian G, et al (2020) Colistin and its role in the Era of antibiotic resistance : an extended review. Emerg Microbes Infect 9:868–885. https://doi.org/10.1080/22221751.2020.1754133
Bialvaei AZ, Samadi Kafil H (2015) Colistin, mechanisms and prevalence of resistance. Curr Med Res Opin 31:707–721. https://doi.org/10.1185/03007995.2015.1018989
Claus BOM, Snauwaert S, Haerynck F et al (2015) Colistin and neurotoxicity: recommendations for optimal use in cystic fibrosis patients. Int J Clin Pharm 37:555–558. https://doi.org/10.1007/s11096-015-0077-4
Dai C, Li J, Lin W et al (2012) Electrophysiology and ultrastructural changes in mouse sciatic nerve associated with colistin sulfate exposure. Toxicol Mech Methods 22:592–596. https://doi.org/10.3109/15376516.2012.704956
Dai C, Li J, Li J (2013) New insight in colistin induced neurotoxicity with the mitochondrial dysfunction in mice central nervous tissues. Exp Toxicol Pathol 65:941–948. https://doi.org/10.1016/j.etp.2013.01.008
Dai C, Tang S, Li J et al (2014) Effects of Colistin on the Sensory Nerve Conduction Velocity and F-wave in Mice. Basic Clin Pharmacol Toxicol 115:577–580. https://doi.org/10.1111/bcpt.12272
Dai C, Xiao X, Zhang Y et al (2020) Curcumin Attenuates Colistin-Induced Peripheral Neurotoxicity in Mice. ACS Infect Dis 6:715–724. https://doi.org/10.1021/acsinfecdis.9b00341
De S, Kuwahara S, Saito A (2014) The endocytic receptor megalin and its associated proteins in proximal tubule epithelial cells. Membranes (basel) 4:333–335. https://doi.org/10.3390/membranes4030333
Dijkmans AC, Wilms EB, Kamerling IMC et al (2015) Colistin: Revival of an Old Polymyxin Antibiotic. Ther Drug Monit 37:419–427. https://doi.org/10.1097/FTD.0000000000000172
Edrees NE, Galal AAA, Abdel Monaem AR et al (2018) Curcumin alleviates colistin-induced nephrotoxicity and neurotoxicity in rats via attenuation of oxidative stress, inflammation and apoptosis. Chem Biol Interact 294:56–64. https://doi.org/10.1016/j.cbi.2018.08.012
Falagas ME, Kasiakou SK (2006) Toxicity of polymyxins: A systematic review of the evidence from old and recent studies. Crit Care 10:1–18. https://doi.org/10.1186/cc3995
Gai Z, Samodelov SL, Kullak-Ublick GA, Visentin M (2019) Molecular Mechanisms of Colistin-Induced Nephrotoxicity. Molecules 24:653. https://doi.org/10.3390/molecules24030653
Gangadhar KN, Adhikari K, Srichana T (2014) Synthesis and evaluation of sodium deoxycholate sulfate as a lipid drug carrier to enhance the solubility, stability and safety of an amphotericin B inhalation formulation. Int J Pharm 471:430–438. https://doi.org/10.1016/j.ijpharm.2014.05.066
Grégoire N, Aranzana-Climent V, Magréault S et al (2017) Clinical Pharmacokinetics and Pharmacodynamics of Colistin. Clin Pharmacokinet 56:1441–1460. https://doi.org/10.1007/s40262-017-0561-1
Hill C, Jain A, Takemoto H et al (2014) A secondary mode of action of polymyxins against Gram- negative bacteria involves the inhibition of NADH-quinone oxidoreductase activity. J Antibiot (tokyo) 67:147–151. https://doi.org/10.1038/ja.2013.111.A
Honoré PM, Jacobs R, Joannes-Boyau O et al (2014) Continuous renal replacement therapy-related strategies to avoid colistin toxicity: A clinically orientated review. Blood Purif 37:291–295. https://doi.org/10.1159/000363495
Kaewpaiboon S, Srichana T (2022) Formulation Optimization and Stability of Polymyxin B Based on Sodium Deoxycholate Sulfate Micelles. J Pharm Sci 000:1–9. https://doi.org/10.1016/j.xphs.2022.02.011
Kalesidis T, Falagas ME (2015) The safety of polymyxin antibiotics Theodoros. Expert Opin Drug Saf 14:1687–1701. https://doi.org/10.1517/14740338.2015.1088520
Karaiskos I, Giamarellou H (2014) Multidrug-resistant and extensively drug-resistant Gram-negative pathogens: Current and emerging therapeutic approaches. Expert Opin Pharmacother 15:1351–1370. https://doi.org/10.1517/14656566.2014.914172
Karaiskos I, Souli M, Galani I, Giamarellou H (2017) Colistin: still a lifesaver for the 21st century? Expert Opin Drug Metab Toxicol 13:59–71. https://doi.org/10.1080/17425255.2017.1230200
Keirstead ND, Wagoner MP, Bentley P et al (2014) Early prediction of polymyxin-induced nephrotoxicity with next-generation urinary kidney injury biomarkers. Toxicol Sci 137:278–291. https://doi.org/10.1093/toxsci/kft247
Kelesidis T, Falagas ME (2015) The safety of polymyxin antibiotics. Expert Opin Drug Saf 14:1687–1701. https://doi.org/10.1517/14740338.2015.1088520
KhumainiMudharBintang MA, Tipmanee V, Srichana T (2023) Colistin sulfate-sodium deoxycholate sulfate micelle formulations; molecular interactions, cell nephrotoxicity and bioactivity. J Drug Deliv Sci Technol 79:104091. https://doi.org/10.1016/j.jddst.2022.104091
Kitada M, Xu J, Ogura Y et al (2020) Manganese superoxide dismutase dysfunction and the pathogenesis of kidney disease. Front Physiol 11:1–16. https://doi.org/10.3389/fphys.2020.00755
Madhumanchi S, Suedee R, Nakpheng T et al (2019) Binding interactions of bacterial lipopolysaccharides to polymyxin B in an amphiphilic carrier ‘sodium deoxycholate sulfate.’ Colloids Surfaces B Biointerfaces 182:110374. https://doi.org/10.1016/j.colsurfb.2019.110374
Madhumanchi S, Suedee R, Kaewpiboon S et al (2020) Effect of sodium deoxycholate sulfate on outer membrane permeability and neutralization of bacterial lipopolysaccharides by polymyxin B formulations. Int J Pharm 581:119265. https://doi.org/10.1016/j.ijpharm.2020.119265
Nation RL, Velkov T, Li J (2014) Colistin and polymyxin B: Peas in a pod, or chalk and cheese? Clin Infect Dis 59:88–94. https://doi.org/10.1093/cid/ciu213
Nazer LH, Anabtawi N (2017) Optimizing colistin dosing: Is a loading dose necessary? Am J Heal Pharm 74:e9–e16. https://doi.org/10.2146/ajhp150876
Neill JO’ (2014) Antimicrobial Resistance: Tackling a crisis for the health and wealth of nations The Review on Antimicrobial Resistance Chaired. Rev Antimicrob Resist 20:1–16
Ortwine JK, Sutton JD, Kaye KS, Pogue JM (2015) Strategies for the safe use of colistin. Expert Rev Anti Infect Ther 13:1237–1247. https://doi.org/10.1586/14787210.2015.1070097
Ozkan G, Ulusoy S, Orem A et al (2013) How does colistin-induced nephropathy develop and can it be treated? Antimicrob Agents Chemother 57:3463–3469. https://doi.org/10.1128/AAC.00343-13
Ozyilmaz E, Ebinc FA, Derici U et al (2011) Could nephrotoxicity due to colistin be ameliorated with the use of N-acetylcysteine? Intensive Care Med 37:141–146. https://doi.org/10.1007/s00134-010-2038-7
Perez F, El Chakhtoura NG, Yasmin M, Bonomo RA (2019) Polymyxins: To combine or not to combine? Antibiotics 8:1–13. https://doi.org/10.3390/antibiotics8020038
Pogue JM, Ortwine JK, Kaye KS (2017) Clinical considerations for optimal use of the polymyxins: A focus on agent selection and dosing. Clin Microbiol Infect 23:229–233. https://doi.org/10.1016/j.cmi.2017.02.023
Sunami R, Sugiyama H, Wang DH et al (2004) Acatalasemia sensitizes renal tubular epithelial cells to apoptosis and exacerbates renal fibrosis after unilateral ureteral obstruction. Am J Physiol - Ren Physiol 286:2–5. https://doi.org/10.1152/ajprenal.00266.2003
Suzuki T, Yamaguchi H, Ogura J et al (2013) Megalin contributes to kidney accumulation and nephrotoxicity of colistin. Antimicrob Agents Chemother 57:6319–6324. https://doi.org/10.1128/AAC.00254-13
Temboot P, Kaewpaiboon S, Tinpun K, et al (2020) Potential of sodium deoxycholate sulfate as a carrier for polymyxin B: Physicochemical properties, bioactivity and in vitro safety. J Drug Deliv Sci Technol 58:101779. https://doi.org/10.1016/j.jddst.2020.101779
Usman F, Ul-Haq Z, Khalil R et al (2017) Pharmacologically Safe Nanomicelles of Amphotericin B With Lipids: Nuclear Magnetic Resonance and Molecular Docking Approach. J Pharm Sci 106:3574–3582. https://doi.org/10.1016/j.xphs.2017.08.013
Usman F, Nopparat J, Javed I, Srichana T (2020) Biodistribution and histopathology studies of amphotericin B sodium deoxycholate sulfate formulation following intratracheal instillation in rat models. Drug Deliv Transl Res 10:59–69. https://doi.org/10.1007/s13346-019-00662-x
Vardakas KZ, Falagas ME (2017) Colistin versus polymyxin B for the treatment of patients with multidrug-resistant Gram-negative infections: a systematic review and meta-analysis. Int J Antimicrob Agents 49:233–238. https://doi.org/10.1016/j.ijantimicag.2016.07.023
Wallace SJ, Li J, Nation RL et al (2008) Subacute toxicity of colistin methanesulfonate in rats: Comparison of various intravenous dosage regimens. Antimicrob Agents Chemother 52:1159–1161. https://doi.org/10.1128/AAC.01101-07
Wang W (2015) Tolerability of hypertonic injectables. Int J Pharm 490:308–315. https://doi.org/10.1016/j.ijpharm.2015.05.069
Yu Z, Qin W, Lin J, et al (2015) Antibacterial mechanisms of polymyxin and bacterial resistance. Biomed Res Int 2015:679109. https://doi.org/10.1155/2015/679109
Zavascki AP, Nation RL (2017) Nephrotoxicity of polymyxins: Is there any difference between colistimethate and polymyxin B? Antimicrob Agents Chemother 61:e02319–16. https://doi.org/10.1128/AAC.02319-16
Acknowledgements
The authors would like to thank Sunisa Kaewpaiboon and Titpawan Nakpheng for assisting in experimental works.
Funding
This work was supported by National Science, Research and Innovation Fund and Prince of Songkla University (PHA6601221S).
Author information
Authors and Affiliations
Contributions
MAKMB, JN, and TS contributed to the study conception and design. Material preparation, data collection and analysis were performed by MAKMB, and JN. The first draft of the manuscript was written by MAKMB, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethics approval
All animal experiments were performed with the approval of the animal ethics committee, Prince of Songkla University (MHESI 68014/1895, Ref. 81/2021).
Consent for publication
All authors consent to the publication of the manuscript.
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bintang, M.A.K.M., Nopparat, J. & Srichana, T. In vivo evaluation of nephrotoxicity and neurotoxicity of colistin formulated with sodium deoxycholate sulfate in a mice model. Naunyn-Schmiedeberg's Arch Pharmacol 396, 3243–3252 (2023). https://doi.org/10.1007/s00210-023-02531-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02531-4