Abstract
The main goal of the present study was to evaluate the influence of the adenosine A1 receptor (A1R) antagonist — DPCPX — on depressive-like behavior in mice, as well as the effect of DPCPX on the activity of imipramine, escitalopram, and reboxetine, each at non-effective doses. The influence of DPCPX on behavior and its influence on the activity of selected antidepressants was evaluated in the forced swim test (FST) and the tail suspension test (TST) in mice. Locomotor activity was measured to verify and exclude false-positive data obtained in the FST and TST. Moreover, serum and brain concentrations of tested antidepressants were determined using HPLC. DPCPX, at doses of 2 and 4 mg/kg, exhibited antidepressant activity in the FST and TST, which was not related to changes in the spontaneous locomotor activity. Co-administration of DPCPX with imipramine, escitalopram, or reboxetine, each at non-active doses, significantly reduced the immobilization period in the FST and TST in mice, which was not due to the increase in locomotor activity. Both antagonists of 5-HT receptors (WAY 100635 and ritanserin) completely antagonized the effect elicited by DPCPX in the behavioral tests. Results of assessment of the nature of the interaction between DPCPX and test drugs show that in the case of DPCPX and imipramine or reboxetine, there were pharmacodynamic interactions, whereas the DPCPX-escitalopram interaction is at least partially pharmacokinetic in nature. Presented outcomes indicate that an inhibition of A1Rs and an increase of monoaminergic transduction in the CNS may offer a novel strategy for the development of antidepressant drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is known that adenosine is involved not only in the regulation of a wide range of behaviors, moods, and emotions (Boison 2008; Cunha et al. 2008; El Yacoubi et al. 2000; Ruby et al. 2010; Asatryan et al. 2011), but also cognitive processes (Kopf et al. 1999) and motor activity (Brockwell and Beninger 1996). Adenosine as a neuromodulator exerts its functions through the activation of four G-protein-coupled adenosine receptors (AR) — A1, A2A, A2B, and A3 (Fredholm et al. 2001, 2005a, b; Jacobson and Gao 2006; Boison 2008). Their roles and pharmacology have been analyzed in detail, with respect to control of transmitter release, modulation of neuronal excitability, and regulation of ion channel function (Fredholm et al. 1999, 2005a, b; Ferré et al. 2010).
Adenosine, adenosine analogs, and adenosine degradation inhibitors, which cause the non-selective AR activation, induce depressive-like behaviors in some animal models of depression (Kulkarni and Mehta 1985; Woodson et al. 1998). On the other hand, much data indicate that a non-selective pharmacological inhibition of adenosine receptors (e.g., administration of methylxanthines such as caffeine, theophylline) may reduce depressive-like behaviors in laboratory animals (Minor et al. 1994a, b; El Yacoubi et al. 2003; Minor and Hanff 2015; Szopa et al. 2016). However, Kaster et al. (2004, 2005a, b, 2007) presented the contrary data, which indicated that the non-selective activation of adenosine receptors decreased the immobilization period in the forced swim test (FST) and tail suspension test (TST) in mice.
Nowadays, due to the high stress accompanying everyday life, the number of patients suffering from mental illness increases annually. As a consequence, psychiatric disorders, including depression, have become one of the biggest problems worldwide (Wittchen et al. 2011; Olesen et al. 2012; WHO 2017). Despite the availability of a wide range of drugs for mental diseases with various mechanisms of action, therapeutic effects are still not optimal, and there is an urgent need to develop alternative therapeutic options. Recently, a particular attention has been given to a relationship between adenosine, adenosine receptors, and processes occurring in the brain under normal and disease conditions (Yamada et al. 2014), so they are perceived as important therapeutic targets (Chen et al. 2013; Sachdeva and Gupta 2013; Yamada et al. 2014; Vincenzi et al. 2016).
Considerable literature indicates that inhibition of adenosine neurotransmission may decrease the symptoms of mental illness, including depression, so it is of interest to determine the effect of A1R antagonist on the activity of commonly used antidepressants. The main goal of this study was to assess the effect of DPCPX — a selective A1-receptor antagonist — on animal behavior during short-term exposure to inescapable and uncontrollable stress. A further goal was to evaluate the influence of DPCPX on the activity of three common antidepressants representing different classes, imipramine — a tricyclic antidepressant (TCA), escitalopram — a selective serotonin reuptake inhibitor (SSRI), and reboxetine — a selective noradrenaline reuptake inhibitor (SNRI). Two behavioral tests widely used to determine antidepressant properties of drugs, the FST, and TST, were used. To verify and exclude false-positive/negative outcomes, spontaneous locomotor activity was measured. In order to elucidate the role of serotoninergic receptors 5-HT1A and 5-HT2 in the actions of tested substances, we used selective antagonists of these receptors — WAY 100635 and ritanserin, respectively. The effect of DPCPX on the level of antidepressants in murine serum and brain homogenates was estimated using high-performance liquid chromatography (HPLC).
Materials and methods
Animals
Adult male albino Swiss mice weighing 25–30 g form licensed breeder (Kołacz, Warsaw, Poland) were used for all experiments. The animals were housed in environmentally controlled rooms (temperature maintained at 21–25 °C and humidity 40–60%) in groups of ten in standard cages with unlimited access to water and food, with a 12 h light/dark cycle. The procedures began after at least a 1-week acclimatization period in the facility and were performed between 8 a.m. and 3 p.m. to minimize circadian influences. All procedures were conducted in accordance with the European Communities Council Directive and Polish legislation acts concerning animal experimentations. The procedures and protocols were approved by the First Local Ethics Committee at the Medical University of Lublin (license no 5/2015).
Drug administration
DPCPX (8-cyclopentyl-1,3-dipropylxanthine, 1, 2, and 4 mg/kg, Sigma-Aldrich, Poznań, Poland) was suspended in a 1% aqueous solution of Tween 80 (POCH S.A., Gliwice, Poland). Imipramine hydrochloride (15 mg/kg, Sigma-Aldrich), reboxetine mesylate (2.5 mg/kg, Ascent Scientific, Cambridge, UK), escitalopram oxalate (2 mg/kg, Sigma-Aldrich), WAY 100635 (0.1 mg/kg, Sigma-Aldrich), and ritanserin (4 mg/kg, Sigma-Aldrich) were dissolved in 0.9% NaCl. All solutions were administrated intraperitoneally (ip) 60 min, whereas DPCPX suspension was injected ip 30 min prior to behavioral testing. The volume of all administrated solutions/suspension was 10 ml/kg. Time of drugs administration was chosen so that the behavioral tests were performed at the point of maximum effect of these substances. The doses and pretreatment schedules were selected on the basis of the literature and prior results (Poleszak 2007; Poleszak et al. 2005, 2011; Szewczyk et al. 2002, 2009; Szopa et al. 2016; Poleszak et al. 2016). In the studies in which the influence of DPCPX on the activity of common antidepressants was examined the non-active doses of DPCPX (1 mg/kg), and tested antidepressants were injected. In turn, in experiments with 5-HT receptor antagonists, an effective dose of DPCPX (2 mg/kg) was used to show whether the serotoninergic receptors 5-HT1A and 5-HT2 are involved in the operation of DPCPX antidepressant-like activity. Control groups received ip injections of saline.
Forced swim test
The FST was carried out according to the method of Porsolt et al. (1977). Each mouse was placed individually for 6 min into a glass cylinder (height 25 cm, diameter 10 cm) containing 15 cm of water at 23–25 °C. After the first 2 min of the test, total duration of immobility was measured. A mouse was judged to be immobile when it ceased struggling and remained floating motionless and making only movements allowing to keep the head just above the surface of water. FST results are presented as the average duration of immobility time (seconds) ± standard error of the mean (SEM) for each experimental group.
Tail suspension test
The TST was carried out according to the method of Steru et al. (1985). Each mouse was suspended individually for 6 min by the tail (2 cm from the end of the tail) using adhesive tape. After the first 2 min of the test, total duration of immobility was measured. A mouse was judged to be immobile when it ceased moving limbs and body, making only movements required to breathe. TST results are presented as the average duration of immobility time (seconds) ± SEM for each experimental group.
Spontaneous locomotor activity
Spontaneous locomotor activity was measured using Opto-Varimex-4 Auto-Track (Columbus Instruments, Columbus, OH, USA) which consist of four cages made of Plexiglas with lids (43 × 43 × 32 cm). The cages were equipped with a set of four infrared emitters and four detectors, which monitor animal movements. Each mouse was placed individually for 6 min into a cage to determine the distance traveled by the animal between 2 and 6 min, which corresponds with the time interval analyzed in the FST and TST. Results obtained in the spontaneous locomotor activity test are presented as the average distance traveled by mice (cm) ± SEM for each experimental group.
Determination of antidepressant drugs levels in serum and brains homogenates of mice
To obtain blood and brain for pharmacokinetic studies, mice were decapitated 60 min after administration of antidepressant drug with or without DPCPX. The blood was collected into Eppendorf tubes and allowed to cloth. Samples were then centrifuged for 10 min at 1000 rpm, and the centrifuged serum were collected into polyethylene tubes and frozen at − 25 °C. Brains, just after decapitation, were dissected from the skulls, rinsed with 0.9% NaCl, and frozen at − 25 °C.
Brain and serum concentrations of antidepressants were assayed by HPLC as described previously (Poleszak et al. 2016; Szopa et al. 2016).
Calibration curves constructed on the basis of the ratios of the peak heights of the tested drugs to the peak heights of the appropriate internal standards versus the known drug concentrations were linear in the tested concentration ranges. No interfering peaks were observed in the chromatograms. Assays were reproducible with low intra- and inter-day variation (a coefficient of variation less than 10%). The extraction efficiencies of the analyzed compounds and internal standards ranged from 66 to 97%. Concentrations of antidepressants were expressed in ng/ml of serum and ng/g of wet brain tissue.
Statistical analysis
Statistical analysis was performed using one-way ANOVA with Dunnett’s post hoc, two-way ANOVA with Bonferroni’s post hoc test, or Student’s t test, depending on the study design. Results are considered statistically significant when the p values were ≤ 0.05.
Results
Forced swim test
DPCPX and antidepressants
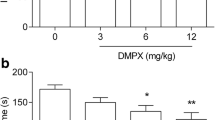
DPCPX, at doses of 1, 2, and 4 mg/kg, was administered to ascertain the dose-effect relationship in the FST (Fig. 1a). DPCPX at doses of 2 and 4 mg/kg, but not 1 mg/kg, caused a significant shortening of the duration of immobility in the FST vs saline-treated group [one-way ANOVA F(3,33) = 9.196; **p < 0.01, ***p < 0.001, p > 0.05 respectively].
The antidepressant activity of DPCPX in the FST (a) and TST (b) in mice. DPCPX and saline were administered ip 30 min prior to the test. The data are presented as the means + SEM. Each experimental group consisted of ten animals. *p < 0.05, **p < 0.01, ***p < 0.001 vs control group (one-way ANOVA followed by Dunnett’s post hoc test)
Imipramine (15 mg/kg) did not statistically significant changes in the FST (p > 0.05). Significant immobility time reduction was noted when DPCPX and imipramine were co-administered in non-effective doses (1 and 15 mg/kg, respectively) (p < 0.0001 vs DPCPX-treated group, p < 0.001 vs imipramine-treated group) (Fig. 2a). A significant effect of imipramine [F(1,36) = 18.33, p = 0.0001], a significant effect of DPCPX [F(1,36) = 10.52, p = 0.0025], and a significant interaction between imipramine and DPCPX [F(1,36) = 7.27, p = 0.0106] were shown in the two-way ANOVA analysis.
Effect of combined administration of DPCPX and antidepressants in the FST in mice. Antidepressants and saline were administered ip 60 min, whereas DPCPX ip 30 min prior to the test. The data are presented as the means + SEM. Each experimental group consisted of ten animals. a. ****p < 0.0001 vs DPCPX-treated group, ***p < 0.001 vs imipramine-treated group. b. *p < 0.05 vs DPCPX-treated group and escitalopram-treated group. c. **p < 0.01 vs DPCPX-treated group and reboxetine-treated group (two-way ANOVA followed by Bonferroni’s post hoc test)
Escitalopram (2 mg/kg) did not cause statistically significant changes in the FST (p > 0.05). Significant immobility time reduction was noted when DPCPX and escitalopram were co-administered in non-effective doses (1 and 2 mg/kg, respectively) (p < 0.05 vs DPCPX-treated group and escitalopram-treated group) (Fig. 2b). A significant effect of escitalopram [F(1,35) = 6.115, p = 0.0184], a significant effect of DPCPX [F(1,35) = 4.435, p = 0.0424], and no interaction between escitalopram and DPCPX were shown in the two-way ANOVA analysis.
Reboxetine (2.5 mg/kg) did not cause statistically significant changes in the FST (p > 0.05). Significant immobility time reduction was noted when DPCPX and reboxetine were co-administered in non-effective doses (1 and 2.5 mg/kg, respectively) (p < 0.01 vs DPCPX-treated group and reboxetine-treated group) (Fig. 2c). No effect of reboxetine [F(1,36) = 3.55, p = 0.0676], a significant effect of DPCPX [F(1,36) = 8636, p = 0.0057], and a significant interaction between reboxetine and DPCPX [F(1,36) = 5.617, p = 0.0233] were shown in the two-way ANOVA analysis.
5-HT receptor antagonists and intrinsic effects of DPCPX
WAY 100635 influenced DPCPX antidepressant-like activity in the FST as demonstrated in Fig. 4a. DPCPX (2 mg/kg), but not WAY 100635 (0.1 mg/kg), produced a statistically significant change in animal behavior in the FST (p < 0.01 and p > 0.05, respectively). The antidepressant-like effect of DPCPX (2 mg/kg) was reduced by the injection of WAY 100635 at a dose of 0.1 mg/kg (p < 0.0001 vs DPCPX-treated group). A significant effect of WAY 100635 [F(1,26) = 15.91; p = 0.0005], no effect of DPCPX [F(1,26) = 3.748; p = 0.0638], and a significant interaction between WAY 100.635 and DPCPX [F(1,26) = 9.273; p = 0.0053] were shown in the two-way ANOVA analysis.
Ritanserin influenced DPCPX antidepressant-like activity in the FST as demonstrated in Fig. 4b. DPCPX (2 mg/kg), but not ritanserin (4 mg/kg), produced a statistically significant change in animal behavior in the FST (p < 0.01 and p > 0.05, respectively). Antidepressant-like effect of DPCPX (2 mg/kg) was reduced by the injection of ritanserin at a dose of 4 mg/kg (p < 0.001 vs DPCPX-treated group). A significant effect of ritanserin [F(1,25) = 16.41; p = 0.0004], no effect of DPCPX [F(1,25) = 3.094; p = 0.0908], and a significant interaction between ritanserin and DPCPX [F(1,25) = 8.298; p = 0.0080] were shown in the two-way ANOVA analysis.
Tail suspension test
DPCPX and antidepressants
DPCPX, at doses of 1, 2, and 4 mg/kg, was administered to ascertain the dose-effect relationship in the TST (Fig. 1b). DPCPX at doses of 2 and 4 mg/kg, but not 1 mg/kg, caused a significant shortening of the duration of the animals’ immobility in the FST vs saline-treated group [one way ANOVA F(3,36) = 6.239, *p < 0.05, **p < 0.01, p > 0.05 respectively].
Imipramine (15 mg/kg) did not cause statistically significant changes in the TST (p > 0.05). Significant immobility time reduction was noted when DPCPX and imipramine were co-administered in non-effective doses (1 and 15 mg/kg, respectively) (p < 0.0001 vs DPCPX-treated group, p < 0.01 vs imipramine-treated group) (Fig. 3a). A significant effect of imipramine [F(1,36) = 21.39, p < 0.001], a significant effect of DPCPX [F(1,36) = 6.255, p = 0.0171], and a significant interaction between imipramine and DPCPX [F(1,36) = 5.217, p = 0.0284] were shown in the two-way ANOVA analysis.
Effect of combined administration of DPCPX and antidepressants in the TST in mice. Antidepressants and saline were administered ip 60 min, whereas DPCPX ip 30 min prior the test. The data are presented as the means + SEM. Each experimental group consisted of ten animals. a. ****p < 0.0001 vs DPCPX-treated group, **p < 0.01 vs imipramine-treated group. b. *p < 0.05 vs DPCPX-treated group and escitalopram-treated group. c. ***p < 0.01 vs DPCPX-treated group, *p < 0.05 vs reboxetine-treated group (two-way ANOVA followed by Bonferroni’s post hoc test)
Escitalopram (2 mg/kg) did not cause statistically significant changes in the TST (p > 0.05). Significant immobility time reduction was noted when DPCPX and escitalopram were co-administered in non-effective doses (1 and 2 mg/kg, respectively) (p < 0.05 vs DPCPX-treated group and escitalopram-treated group) (Fig. 3b). A significant effect of escitalopram [F(1,36) = 6.006, p = 0.0192], no effect of DPCPX [F(1,36) = 3.179, p = 0.0830], and no interaction between escitalopram and DPCPX [F(1,36) = 2.383, p = 0.1314] were shown in the two-way ANOVA analysis.
Reboxetine (2.5 mg/kg) did not cause statistically significant changes in the TST (p > 0.05). Significant immobility time reduction was noted when DPCPX and reboxetine were co-administered in non-effective doses (1 and 2.5 mg/kg, respectively) (p < 0.001 vs DPCPX-treated group, p < 0.01 vs reboxetine-treated group) (Fig. 3c). A significant effect of reboxetine [F(1,36) = 20.08, p < 0,001], no effect of DPCPX [F(1,36) = 4.072, p = 0.0511], and no interaction between reboxetine and DPCPX [F(1,36) = 3.262, p = 0.0793] were shown in the two-way ANOVA analysis.
5-HT receptor antagonists and intrinsic effects of DPCPX
WAY 100635 influenced DPCPX antidepressant-like activity in the TST as demonstrated in Fig. 4c. DPCPX (2 mg/kg), but not WAY 100635 (0.1 mg/kg), produced a statistically significant change in animal behavior in the FST (p < 0.01 and p > 0.05, respectively). The antidepressant-like effect of DPCPX (2 mg/kg) was reduced by the injection of WAY 100635 at a dose of 0.1 mg/kg (p < 0.001 vs DPCPX-treated group). A significant effect of WAY 100635 [F(1,28) = 10.99; p = 0.0025], no effect of DPCPX [F(1,28) = 3.567; p = 0.0693], and a significant interaction between WAY 100635 and DPCPX [F(1,28) = 7.058; p = 0.0129] were shown in the two-way ANOVA analysis.
Effect of combined administration of DPCPX and selective antagonists of serotonin receptors 5-HT1A and 5-HT2 in the FST (a, b) and TST (c, d) in mice. WAY 100635, ritanserin, and saline were administered ip 60 min, whereas DPCPX ip 30 min prior to the test. The data are presented as the means + SEM. Each experimental group consisted of ten animals. **p < 0.01 vs control group, ***p < 0.001, ****p < 0.0001 vs DPCPX-treated group (two-way ANOVA followed by Bonferroni’s post hoc test)
Ritanserin influenced DPCPX antidepressant-like activity in the TST as demonstrated in Fig. 4d. DPCPX (2 mg/kg), but not ritanserin (4 mg/kg), produced a statistically significant change in animal behavior in the TST (p < 0.01 and p > 0.05, respectively). The antidepressant-like effect of DPCPX (2 mg/kg) was reduced by the injection of ritanserin at a dose of 4 mg/kg (p < 0.0001 vs DPCPX-treated group). A significant effect of ritanserin [F(1,28) = 15.55; p = 0.0005], no effect of DPCPX [F(1,28) = 0.9525; p = 0.3374], and a significant interaction between ritanserin and DPCPX [F(1,28) = 12.62; p = 0.0014] were shown in the two-way ANOVA analysis.
Spontaneous locomotor activity
The effect of DPCPX (1, 2, and 4 mg/kg) and combined administration of DPCPX and the tested antidepressants on spontaneous locomotor activity in mice is shown in Tables 1 and 2. DPCPX (1, 2, and 4 mg/kg), imipramine (15 mg/kg), escitalopram (2 mg/kg), reboxetine (2.5 mg/kg), and WAY 100635 (0.1 mg/kg) given alone or in combination had no statistically significant effects on locomotor activity in mice. A single injection of ritanserin (4 mg/kg) and combined administration of DPCPX with ritanserin significantly (p < 0.001) shortened the locomotor activity in mice.
The two-way ANOVA demonstrated: (A) no effect of imipramine [F(1,28) = 0.1096, p = 0.7431], no effect of DPCPX [F(1,28) = 0.2924, p = 0.5930], and no interaction [F(1,28) = 0.2503, p = 0.6208]. (B) no effect of escitalopram [F(1,28) = 0.7513; p = 0.3934], no effect of DPCPX [F(1,28) = 0.0001025, p = 0.9920], and no interaction [F(1,28) = 0.001185, p = 0.9728]. (C) no effect of reboxetine [F(1,28) = 0.08898, p = 0.7677], no effect of DPCPX [F(1,28) = 0.04666, p = 0.8305], and no interaction [F(1,28) = 0.06826, p = 0.7958]. (D) no effect of WAY 100635 [F(1,28) = 0.1493, p = 0.7021], no effect of DPCPX [F(1,28) = 0.001156; p = 0.9731], and no interaction [F(1,28) = 0.1178, p = 0.7340]. (E) a significant effect of ritanserin [F(1,27) = 59.45, p < 0,001], no effect of DPCPX [F(1,27) = 0.009954, p = 0.9213], and no interaction [F(1,27) = 0.2720, p = 0.6062].
Pharmacokinetic studies
The effect of DPCPX on serum and brain concentrations of antidepressants in mice is shown in Table 3. In the case of combined administration of DPCPX with imipramine and reboxetine, no significant changes in drug concentration were observed in murine serum and brain homogenates. DPCPX increased the concentration of escitalopram (t test p < 0.01) in brain tissue without significant changes in serum (t test p > 0.05).
Discussion
DPCPX and antidepressant drug activity in the FST and TST
Behavioral despair and learned helplessness are typical symptoms of depressive disorders, and adenosine systems may be involved. Outcomes obtained by Woodson et al. suggested that an essential constituent in behavior induced by stress are adenosine and its receptor activation (Woodson et al. 1998). Moreover, Minor et al. indicated that depression-like effects in rodents are induced by administration either of a non-selective AR agonist (NECA) or a highly selective A1R agonist (R-PIA) (Minor et al. 1994b). Antidepressant-like activity of the non-selective ARs antagonist caffeine has been demonstrated in the FST and TST, and such effects were comparable with that of TCAs (Enríquez-Castillo et al. 2008; Vieira et al. 2008; Gan 2009; Szopa et al. 2016).
In the present study, the antidepressant-like effect of DPCPX in the FST and TST in mice has been shown. Doses of 2 and 4 mg/kg produced a significant reduction in the immobility time of animals in carried out behavioral tests, whereas the lowest dose of DPCPX − 1 mg/kg — did not exhibit antidepressant-like activity. The highest density of A1Rs is found in the brain, especially in the hippocampus, cortex, and striatum (Ochiishi et al. 1999; Hoyer et al. 2002; Hannon and Hoyer 2008; Wei et al. 2011). A1Rs stimulation leads to an inhibition of several neurotransmitter release and a reduction in postsynaptic excitability (Dunwiddie and Masino 2001). Inversely, an inhibition of these receptors causes a stimulation of neurotransmitter release (e.g., ACh, 5-HT, NA, DA) (Nestler et al. 2002). Shortening of the immobility duration in the FST and TST after administration of the selective A1R antagonist DPCPX, which has a xanthine structure, is probably the result of increased serotonergic, noradrenergic, and dopaminergic transduction (Müller and Scior 1993; Ferré et al. 1996; Müller and Stein 1996; Fredholm et al. 2005b; Górska and Gołembiowska 2015). These outcomes seem to be in disagreement to data that enhancement of adenosine is a possible treatment strategy for depression (Kaster et al. 2004).
The present findings demonstrate that DPCPX affects the action of imipramine, escitalopram, and reboxetine, and these are novel observations. Simultaneous administration of DPCPX and these agents at non-active doses resulted in a statistically significant reduction in the immobility times in either the FST or TST. Also, Herbet et al. have shown recently that co-administration of other selective A1R antagonist — CPT (8-cyclopentyl-1,3-dimethylxanthine, 3 mg/kg) — with imipramine at a non-active doses resulted in a statistically significant reduction in the immobility times during the behavioral test using short-term exposure to inescapable and/or uncontrollable stress (Herbet et al. 2018). Synergism of antidepressant actions was also observed using concomitant administration of TCAs, SSRIs, and SNRIs and the non-selective AR antagonist — caffeine — at ineffective doses in the mice FST (Szopa et al. 2016). The excitation of AR by adenosine, adenosine analogs, and selective AR agonists modulates serotonergic and dopaminergic neurotransmission (Regenold and Illes 1990; Okada et al. 2001; Yamato et al. 2002), and consequently decreases levels of ACh, 5-HT, and DA in the CNS (Okada et al. 2002). Furthermore, these substances suppress medicinal effects of commonly used antidepressants (Barcellos et al. 1998). Drugs which increase levels of monoamines such as 5-HT, NA, and DA in the CNS play a vital role in antidepressant therapy. DPCPX selectively influences A1Rs, which are presented on serotonergic neurons in the locus coeruleus (Regenold and Illes 1990) and the dorsal raphe nucleus (Mössner et al. 2000). The non-selective and selective blockage of A1Rs inhibits effects of endogenous adenosine and cause the opposite effect with regard to the NA and 5-HT transduction (Müller and Scior 1993; Müller and Stein 1996; Fredholm et al. 2005a, b). Preclinical and clinical studies show that antidepressant treatment affects ARs and modify behavioral responses. For example, TCAs are capable of attaching to ARs causing a reduction of extracellular adenosine level in the CNS synapses (Barcellos et al. 1998). All tested antidepressant agents modulate monoaminergic transmission: imipramine non-selectively inhibits neuronal NA and 5-HT reuptake (Sulser et al. 1962), escitalopram is the selective 5-HT reuptake inhibitor (Bræstrup and Sanchez 2004), while reboxetine selectively blocks neuronal reuptake of NA in the CNS (Hajós et al. 2004). The above effects may explain synergy observed between DPCPX and imipramine, escitalopram, and reboxetine in the present study.
5-HT receptor antagonists and intrinsic effects of DPCPX
The serotonin 5-HT1A receptor is an autoreceptor bestead on serotonergic neurons in the raphe nuclei (Sprouse and Aghajanian 1988) and is also found as a postsynaptic receptor localized in the hippocampus and amygdala (Chalmers and Watson 1991). In turn, the 5-HT2 receptor is located mainly on the postsynaptic serotonergic neurons in forebrain (López-Giménez et al. 1997). A1Rs are found in close proximity of 5-HT1 and 5-HT2A receptors. A high level of A1R expression was found in the cerebral cortex, hippocampus, cerebellum, striatum, and in the thalamic nuclei (Hoyer et al. 2002; Hannon and Hoyer 2008). This arrangement of receptors may indicate their mutual interaction. Due to the high probability that DPCPX affects serotonergic transmission based on common localization of receptors, an attempt was made to elucidate the involvement of serotonin 5-HT1A and 5-HT2A/2C receptor in its action. In our study, we determined whether pharmacological antagonism of 5-HT1A or 5-HT2 receptors (WAY 100635 and ritanserin, respectively) would modulate DPCPX activity in the FST and TST. Results show that WAY 100635 (0.1 mg/kg) and ritanserin (4 mg/kg) completely antagonized the effect of DPCPX (2 mg/kg) in both tests. Antidepressant-like activity of DPCPX in the FST and TST appears to be dependent, at least in part, on the serotonergic transmission via 5-HT1A and 5-HT2A/C. Supporting our proposal, results obtained by Detke et al. (1995), Redrobe et al. (1996), Redrobe and Bourin (1997), and Abert and Lemonde (2004) which demonstrated that 5-HT1A and 5-HT2A/C receptors participate in SSRIs antidepressant-like effect in rodent screening tests.
Spontaneous locomotor activity
Since it is widely known that the antidepressant-like effect in the FST and TST may be evoked by the substances which induce hyperactivity, the influence of DPCPX and its combination with antidepressants on the spontaneous locomotor activity was evaluated. Shortening of the duration of immobility observed in presented studies was not associated with the increase of spontaneous locomotor activity. This is consistent with the results in Suzuki et al., which demonstrated that A1-selective antagonists did not increase spontaneous locomotion (Suzuki et al. 1993). Only ritanserin combined with DPCPX inhibited spontaneous locomotor activity. In other studies, similar effects on locomotor activity were observed after administration of ritanserin and compounds with potential antidepressant-like activity (Szewczyk et al. 2009; Poleszak et al. 2016).
Pharmacokinetic studies
Pharmacokinetic investigation in the present study allowed us to assess concentrations of imipramine, escitalopram, and reboxetine in mice blood and brain after their combined administration with DPCPX and was aimed at determining drug-drug interactions involving changes in drug disposition. The present work is the first study in which such an attempt was made. There is no information about DPCPX pharmacokinetics in rodents or humans. Because DPCPX is a xanthine derivative (Müller and Jacobson 2011), it is likely that its metabolism is similar to that of other xanthines, e.g., caffeine and theophylline. The role of cytochromes P450 (CYPs) in the oxidative biotransformation of most drugs is well documented (Caccia 1998; Nelson et al. 2004; Guengerich 2008; Zanger et al. 2008). CYP1A2, the main isoenzyme responsible for the metabolism of xanthines, including caffeine, is also involved in metabolism of commonly used antidepressant drugs (Caccia 1998). Based on HPLC analysis of murine blood and brain tissue, we found that DPCPX does not statistically significantly affect the concentrations of imipramine, its active metabolite desipramine, and reboxetine. In the case of DPCPX and escitalopram, a significant increase in levels of antidepressant drug in the brain but no changes in the serum were noted. These outcomes suggest that DPCPX-impramine and DPCPX-reboxetine interaction are probably due to changes at the cellular level, so are pharmacodynamic in nature. Augmentation of escitalopram levels in the brain may be due to the facilitated transport of this drug through the blood-brain barrier (Pardridge 2005, 2007) after concomitant injection with DPCPX. The increase in antidepressant-like activity of escitalopram observed in the behavioral tests may be partly the result of pharmacokinetic interaction between DPCPX and escitalopram.
Conclusions
In summary, we demonstrated that DPCPX produced an antidepressant-like effect. Furthermore, DPCPX significantly augmented the antidepressant-like potential of a TCA, SSRI, and SNRI without affecting spontaneous locomotor activity. The simultaneous blockage of A1Rs and increase of monoamine transduction in the CNS may offer an alternative target in the development of new options for the pharmacological treatment in patients with depression. In the near future, we are planning to perform studies using chronic concomitant administration of A1Rs antagonists and commonly used antidepressants.
References
Abert PR, Lemonde S (2004) 5-HT1A receptors, gene repression, and depression: guilt by association. Neuroscientist 10:575–593
Asatryan L, Nam HW, Lee MR, Thakkar MM, Saeed DM, Davies DL, Choi DS (2011) Implication of the purinergic system in alcohol use disorders. Alcohol Clin Exp Res 35:584–594
Barcellos CK, Schetinger MR, Dias RD, Sarkis JJ (1998) In vitro effect of central nervous system active drugs on the ATPase–ADPase activity and acetylcholinesterase activity from cerebral cortex of adult rats. Gen Pharmacol 31:563–567
Boison D (2008) Adenosine as a neuromodulator in neurological diseases. Curr Opin Pharmacol 8:2–7
Bræstrup C, Sanchez C (2004) Escitalopram: a unique mechanism of action. Int J Psychiatry Clin Pract 8(Suppl 1):11–13
Brockwell NT, Beninger RJ (1996) The differential role of A1 and A2 adenosine receptor subtypes in locomotor activity and place conditioning in rats. Behav Pharmacol 7:373–383
Caccia S (1998) Metabolism of the newer antidepressants. An overview of the pharmacological and pharmacokinetic implications. Clin Pharmacokinet 34:281–302
Chalmers DT, Watson SJ (1991) Comparative anatomical distribution of 5-HT1A receptor mRNA and 5-HT1A binding in rat brain – a combined in situ hybridisation/in vitro receptor autoradiographic study. Brain Res 561:51–60
Chen JF, Eltzschig HK, Fredholm BB (2013) Adenosine receptors as drug targets – what are the challenges? Nat Rev Drug Discov 12:265–286
Cunha RA, Ferré S, Vaugeois JM, Chen JF (2008) Potential therapeutic interest of adenosine A2A receptors in psychiatric disorders. Curr Pharm Des 14:1512–1524
Detke MJ, Rickels M, Lucki I (1995) Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology 121:66–72
Dunwiddie TV, Masino SA (2001) The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci 24:31–55
El Yacoubi M, Ledent C, Parmentier M, Costentin J, Vaugeois JM (2000) The anxiogenic-like effect of caffeine in two experimental procedures measuring anxiety in the mouse is not shared by selective A(2A) adenosine receptor antagonists. Psychopharmacology 148:153–163
El Yacoubi M, Costentin J, Vaugeois JM (2003) Adenosine A2A receptors and depression. Neurology 61:S82–S87
Enríquez-Castillo A, Alamilla J, Barral J, Gourbière S, Flores-Serrano AG, Góngora-Alfaro JL, Pineda JC (2008) Differential effects of caffeine on the antidepressant-like effect of amitriptyline in female rat subpopulations with low and high immobility in the forced swimming test. Physiol Behav 94:501–509
Ferré S, Popoli P, Tinner-Staines B, Fuxe K (1996) Adenosine A1 receptor-dopamine D1 receptor interaction in the rat limbic system: modulation of dopamine D1 receptor antagonist binding sites. Neurosci Lett 208:109–112
Ferré S, Lluís C, Justinova Z, Quiroz C, Orru M, Navarro G, Canela EI, Franco R, Goldberg SR (2010) Adenosine – cannabinoid receptor interactions. Implications for striatal function. Br J Pharmacol 160:443–453
Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau EE (1999) Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev 51:83–133
Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J (2001) International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 53:527–552
Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM (2005a) Adenosine and brain function. Int Rev Neurobiol 63:191–270. https://doi.org/10.1016/S0074-7742:-3
Fredholm BB, Chen JF, Masino SA, Vaugeois JM (2005b) Actions of adenosine at its receptors in the CNS: insights from knockouts and drugs. Annu Rev Pharmacol Toxicol 45:385–412. https://doi.org/10.1146/annurev.pharmtox.45.120403.095731.:385-412
Gan SH (2009) Dual effects of low and high dose caffeine. Lat Am J Pharm 28:465–469
Górska AM, Gołembiowska K (2015) The role of adenosine A1 and A2A receptors in the caffeine effect on MDMA-induced DA and 5-HT release in the mouse striatum. Neurotox Res 27:229–245
Guengerich FP (2008) Cytochrome p450 and chemical toxicology. Chem Res Toxicol 21:70–83
Hajós M, Fleishaker JC, Filipiak-Reisner JK, Brown MT, Wong EH (2004) The selective norepinephrine reuptake inhibitor antidepressant reboxetine: pharmacological and clinical profile. CNS Drug Rev 10:23–44
Hannon J, Hoyer D (2008) Molecular biology of 5-HT receptors. Behav Brain Res 195:198–213
Herbet M, Szopa A, Serefko A, Wośko S, Gawrońska-Grzywacz M, Izdebska M, Piątkowska-Chmiel I, Betiuk P, Poleszak E, Dudka J (2018) 8-Cyclopentyl-1,3-dimethylxanthine enhances effectiveness of antidepressant in behavioral tests and modulates redox balance in the cerebral cortex of mice. Saudi Pharm J. https://doi.org/10.1016/j.jsps.2018.02.021
Hoyer D, Hannon JP, Martin GR (2002) Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav 71:533–554
Jacobson KA, Gao ZG (2006) Adenosine receptors as therapeutic targets. Nat Rev Drug Discov 5:247–264
Kaster MP, Rosa AO, Rosso MM, Goulart EC, Santos AR, Rodrigues AL (2004) Adenosine administration produces an antidepressant-like effect in mice: evidence for the involvement of A1 and A2A receptors. Neurosci Lett 355:21–24
Kaster MP, Rosa AO, Santos AR, Rodrigues AL (2005a) Involvement of nitric oxide-cGMP pathway in the antidepressant-like effects of adenosine in the forced swimming test. Int J Neuropsychopharmacol 8:601–606
Kaster MP, Santos AR, Rodrigues AL (2005b) Involvement of 5-HT1A receptors in the antidepressant-like effect of adenosine in the mouse forced swimming test. Brain Res Bull 67:53–61
Kaster MP, Budni J, Santos AR, Rodrigues AL (2007) Pharmacological evidence for the involvement of the opioid system in the antidepressant-like effect of adenosine in the mouse forced swimming test. Eur J Pharmacol 576:91–98
Kopf SR, Melani A, Pedata F, Pepeu G (1999) Adenosine and memory storage: effect of A(1) and A(2) receptor antagonists. Psychopharmacology 146:214–219
Kulkarni SK, Mehta AK (1985) Purine nucleoside – mediated immobility in mice: reversal by antidepressants. Psychopharmacology 85:460–463
López-Giménez JF, Mengod G, Palacios JM, Vilaró MT (1997) Selective visualization of rat brain 5-HT2A receptors by autoradiography with [3H]MDL 100,907. Naunyn Schmiedeberg's Arch Pharmacol 356:446–454
Minor TR, Hanff TC (2015) Adenosine signaling in reserpine-induced depression in rats. Behav Brain Res 286:184–191. https://doi.org/10.1016/j.bbr.2015.02.032
Minor TR, Chang WC, Winslow JL (1994a) Stress and adenosine: I. Effect of methylxanthine and amphetamine stimulants on learned helplessness in rats. Behav Neurosci 108:254–264
Minor TR, Winslow JL, Chang WC (1994b) Stress and adenosine: II. Adenosine analogs mimic the effect of inescapable shock on shuttle-escape performance in rats. Behav Neurosci 108:265–276
Mössner R, Albert D, Persico AM, Hennig T, Bengel D, Holtmann B, Schmitt A, Keller F, Simantov R, Murphy D, Seif I, Deckert J, Lesch KP (2000) Differential regulation of adenosine A(1) and A(2A) receptors in serotonin transporter and monoamine oxidase A-deficient mice. Eur Neuropsychopharmacol 10(6):4895–4893
Müller CE, Jacobson KA (2011) Xanthines as adenosine receptor antagonists. Handb Exp Pharmacol 200:151–199. https://doi.org/10.1007/978-3-642-13443-2_6
Müller CE, Scior T (1993) Adenosine receptors and their modulators. Pharm Acta Helv 68:77–111
Müller CE, Stein B (1996) Adenosine receptor antagonists: structures and potential therapeutic applications. Curr Pharm Des 2:501–530
Nelson DR, Zeldin DC, Hoffman SM, Maltais LJ, Wain HM, Nebert DW (2004) Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics 14:1–18
Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM (2002) Neurobiology of depression. Neuron 34:13–25
Ochiishi T, Chen L, Yukawa A, Saitoh Y, Sekino Y, Arai T, Nakata H, Miyamoto H (1999) Cellular localization of adenosine A1 receptors in rat forebrain: immunohistochemical analysis using adenosine A1 receptor-specific monoclonal antibody. J Comp Neurol 411:301–316
Okada M, Nutt DJ, Murakami T, Zhu G, Kamata A, Kawata Y, Kaneko S (2001) Adenosine receptor subtypes modulate two major functional pathways for hippocampal serotonin release. J Neurosci 21:628–640
Okada M, Zhu G, Yoshida S, Iwasa H, Kaneko S (2002) Mechanisms of interaction between adenosine receptor subtypes on hippocampal serotonin release. Nihon Shinkei Seishin Yakurigaku Zasshi 22:61–69
Olesen J, Gustavsson A, Svensson M, Wittchen HU, Jonsson B (2012) The economic cost of brain disorders in Europe. Eur J Neurol 19:155–162
Pardridge WM (2005) The blood-brain barrier: bottleneck in brain drug development. Neuro Rx 2:3–14
Pardridge WM (2007) Blood-brain barrier delivery. Drug Discov Today 12:54–61
Poleszak E, Wlaź P, Szewczyk B, Kedzierska E, Wyska E, Librowski T, Szymura-Oleksiak J, Fidecka S, Pilc A, Nowak G (2005) Enhancement of antidepressant-like activity by joint administration of imipramine and magnesium in the forced swim test: Behavioral and pharmacokinetic studies in mice. Pharmacol Biochem Behav 81(3):524–529
Poleszak E (2007) Modulation of antidepressant-like activity of magnesium by serotonergic system. J Neural Transm (Vienna) 114(9):1129–1134
Poleszak E, Wlaź P, Szewczyk B, Wlaź A, Kasperek R, Wróbel A, Nowak G (2011) A complex interaction between glycine/NMDA receptors and serotonergic/noradrenergic antidepressants in the forced swim test in mice. J Neural Transm (Vienna) 118(11):1535–1546
Poleszak E, Stasiuk W, Szopa A, Wyska E, Serefko A, Oniszczuk A, Wośko S, Świąder K, Wlaź P (2016) Traxoprodil, a selective antagonist of the NR2B subunit of the NMDA receptor, potentiates the antidepressant-like effects of certain antidepressant drugs in the forced swim test in mice. Metab Brain Dis 31(4):803–814. https://doi.org/10.1007/s11011-016-9810-5
Porsolt RD, Bertin A, Jalfre M (1977) Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 229:327–336
Redrobe JP, Bourin M (1997) Partial role of 5-HT2 and 5-HT3 receptors in the activity of antidepressants in the mouse forced swimming test. Eur J Pharmacol 1;325(2-3):129–135
Redrobe JP, MacSweeney CP, Bourin M (1996) The role of 5-HT1A and 5-HT1B receptors in antidepressant drug actions in the mouse forced swimming test. Eur J Pharmacol 318:213–220
Regenold JT, Illes P (1990) Inhibitory adenosine A1-receptors on rat locus coeruleus neurones. An intracellular electrophysiological study. Naunyn Schmiedeberg's Arch Pharmacol 341:225–231
Ruby CL, Adams CA, Knight EJ, Nam HW, Choi DS (2010) An essential role for adenosine signaling in alcohol abuse. Curr Drug Abuse Rev 3:163–174
Sachdeva S, Gupta M (2013) Adenosine and its receptors as therapeutic targets: an overview. Saudi Pharm J 21:245–253
Sprouse JS, Aghajanian GK (1988) Responses of hippocampal pyramidal cells to putative serotonin 5-HT1A and 5-HT1B agonists: a comparative study with dorsal raphe neurons. Neuropharmacology 27:707–715
Steru L, Chermat R, Thierry B, Simon P (1985) The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology 85:367–370
Sulser F, Watts J, Brodie BB (1962) On the mechanism of antidepressant action of imipramine-like drugs. Ann N Y Acad Sci 96:279–288
Suzuki F, Shimada J, Shiozaki S, Ichikawa S, Ishii A, Nakamura J, Nonaka H, Kobayashi H, Fuse E (1993) Adenosine A1 antagonists. 3. Structure-activity relationships on amelioration against scopolamine- or N6-((R)-phenylisopropyl)adenosine-induced cognitive disturbance. J Med Chem 36:2508–2518
Szewczyk B, Brański P, Wierońska JM, Pałucha A, Pilc A, Nowak G (2002) Interaction of zinc with antidepressants in the forced swimming test in mice. Pol J Pharmacol 54(6):681–685
Szewczyk B, Poleszak E, Wlaź P, Wróbel A, Blicharska E, Cichy A, Dybała M, Siwek A, Pomierny-Chamioło L, Piotrowska A, Brański P, Pilc A, Nowak G (2009) The involvement of serotonergic system in the antidepressant effect of zinc in the forced swim test. Prog Neuro-Psychopharmacol Biol Psychiatry 33(2):323–329. https://doi.org/10.1016/j.pnpbp.2008.12.011
Szopa A, Poleszak E, Wyska E, Serefko A, Wośko S, Wlaź A, Pierog M, Wrobel A, Wlaź P (2016) Caffeine enhances the antidepressant-like activity of common antidepressant drugs in the forced swim test in mice. Naunyn Schmiedeberg's Arch Pharmacol 389:211–221
Vieira C, De Lima TC, Carobrez AP, Lino-de-Oliveira C (2008) Frequency of climbing behavior as a predictor of altered motor activity in rat forced swimming test. Neurosci Lett 445:170–173
Vincenzi F, Ravani A, Pasquini S, Merighi S, Gessi S, Romagnoli R, Baraldi PG, Borea PA, Varani K (2016) Positive allosteric modulation of A1 adenosine receptors as a novel and promising therapeutic strategy for anxiety. Neuropharmacology 111:283–292. https://doi.org/10.1016/j.neuropharm.2016.09.015
Wei CJ, Li W, Chen JF (2011) Normal and abnormal functions of adenosine receptors in the central nervous system revealed by genetic knockout studies. Biochim Biophys Acta 1808(5):1358–1379. https://doi.org/10.1016/j.bbamem.2010.12.018
WHO (2017) Depression and Other Common Mental Disorders: Global Health Estimates. Geneva: World Health Organization. Licence: CC BY-NC-SA 3.0 IGO
Wittchen HU, Jacobi F, Rehm J, Gustavsson A, Svensson M, Jonsson B, Olesen J, Allgulander C, Alonso J, Faravelli C, Fratiglioni L, Jennum P, Lieb R, Maercker A, van Os J, Preisig M, Salvador-Carulla L, Simon R, Steinhausen HC (2011) The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol 21:655–679
Woodson JC, Minor TR, Job RF (1998) Inhibition of adenosine deaminase by erythro-9-(2-hydroxy-3-nonyl)adenine (EHNA) mimics the effect of inescapable shock on escape learning in rats. Behav Neurosci 112:399–409
Yamada K, Kobayashi M, Kanda T (2014) Involvement of adenosine A2A receptors in depression and anxiety. Int Rev Neurobiol 119:373–393. https://doi.org/10.1016/B978-0-12-801022-8.00015-5.:373-393
Yamato T, Yamasaki S, Misumi Y, Kino M, Obata T, Aomine M (2002) Modulation of the stress response by coffee: an in vivo microdialysis study of hippocampal serotonin and dopamine levels in rat. Neurosci Lett 332:87–90
Zanger UM, Turpeinen M, Klein K, Schwab M (2008) Functional pharmacogenetics/genomics of human cytochromes P450 involved in drug biotransformation. Anal Bioanal Chem 392:1093–1108
Acknowledgments
The authors wish to thank the Chair and Department of Hygiene of Medical University in Lublin for access to an animal activity meter Opto-Varimex-4 Auto-Track.
Funding
This study was supported by Funds for Statutory Activity of Medical University of Lublin, Poland.
Author information
Authors and Affiliations
Contributions
EP and ASz conceived and designed research. ASz, KB, SW, and AS conducted the behavioral experiments. EW and KŚ conducted the pharmacokinetic studies. UD, JD, and AW analyzed the data. AWl and PW interpreted the results. ASz and KB wrote the manuscript. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures were conducted in accordance with the European Communities Council Directive of 22 September 2010 (2010/63/EU) and Polish legislation acts concerning animal experimentations. The experimental procedures and protocols were approved by the First Local Ethics Committee at the Medical University of Lublin.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Szopa, A., Poleszak, E., Bogatko, K. et al. DPCPX, a selective adenosine A1 receptor antagonist, enhances the antidepressant-like effects of imipramine, escitalopram, and reboxetine in mice behavioral tests. Naunyn-Schmiedeberg's Arch Pharmacol 391, 1361–1371 (2018). https://doi.org/10.1007/s00210-018-1551-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-018-1551-z