Abstract
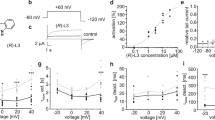
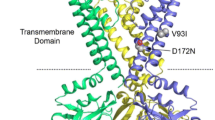
The anticholinergic antiparkinson drug orphenadrine is an antagonist at central and peripheral muscarinic receptors. Orphenadrine intake has recently been linked to QT prolongation and Torsade-de-Pointes tachycardia. So far, inhibitory effects on I Kr or cloned HERG channels have not been examined. HERG channels were heterologously expressed in a HEK 293 cell line and in Xenopus oocytes and HERG current was measured using the whole cell patch clamp and the double electrode voltage clamp technique. Orphenadrine inhibits cloned HERG channels in a concentration dependent manner, yielding an IC50 of 0.85 μM in HEK cells. Onset of block is fast and reversible upon washout. Orphenadrine does not alter the half-maximal activation voltage of HERG channels. There is no shift of the half-maximal steady-state-inactivation voltage. Time constants of direct channel inactivation are not altered significantly and there is no use-dependence of block. HERG blockade is attenuated significantly in mutant channels lacking either of the aromatic pore residues Y652 and F656. In conclusion, we show that the anticholinergic agent orphenadrine is an antagonist at HERG channels. These results provide a novel molecular basis for the reported proarrhythmic side effects of orphenadrine.







Similar content being viewed by others
References
Abbott GW, Sesti F, Splawski I, Buck ME, Lehmann MH, Timothy KW, Keating MT, Goldstein SA (1999) MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell 97:175–187
Altomare C, Terragni B, Brioschi C, Milanesi R, Pagliuca C, Viscomi C, Moroni A, Baruscotti M, DiFrancesco D (2003) Heteromeric HCN1–HCN4 channels: a comparison with native pacemaker channels from the rabbit sinoatrial node. J Physiol 549:347–359
Anantharam A, Lewis A, Panaghie G, Gordon E, McCrossan ZA, Lerner DJ, Abbott GW (2003) RNA interference reveals that endogenous Xenopus MinK-related peptides govern mammalian K+ channel function in oocyte expression studies. J Biol Chem 278:11739–11745
Barish ME (1983) A transient calcium-dependent chloride current in the immature Xenopus oocyte. J Physiol 342:309–325
Brocks DR (1999) Anticholinergic drugs used in Parkinson’s disease: an overlooked class of drugs from a pharmacokinetic perspective. J Pharm Pharmacol Sci 2:39–46
Choe H, Nah KH, Lee SN, Lee HS, Lee HS, Jo SH, Leem CH, Jang YJ (2006) A novel hypothesis for the binding mode of HERG channel blockers. Biochem Biophys Res Commun 344:72–78
Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT (1995) A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell 80:795–803
Danze LK, Langdorf MI (1991) Reversal of orphenadrine-induced ventricular tachycardia with physostigmine. J Emerg Med 9:453–457
Dilaveris P, Pantazis A, Vlasseros J, Gialafos J (2001) Non-sustained ventricular tachycardia due to low-dose orphenadrine. Am J Med 111:418–419
Ellison T (1972) Metabolic studies of 3 H-orphenadrine citrate in the rat, dog and rhesus monkey. Arch Int Pharmacodyn Ther 195:213–230
Henderson RA, Lane S, Henry JA (1991) Life-threatening ventricular arrhythmia (torsades de pointes) after haloperidol overdose. Hum Exp Toxicol 10:59–62
Kiehn J, Lacerda AE, Brown AM (1999) Pathways of HERG inactivation. Am J Physiol 277:H199–H210
Kiesecker C, Zitron E, Lück S, Bloehs R, Scholz EP, Kathöfer S, Thomas D, Kreye VAW, Katus HA, Schoels W, Karle CA, Kiehn J (2004) Class Ia anti-arrhythmic drug ajmaline blocks HERG potassium channels: mode of action. Naunyn Schmiedebergs Arch Pharmacol 370:423–435
Kornhuber J, Parsons CG, Hartmann S, Retz W, Kamolz S, Thome J, Riederer P (1995) Orphenadrine is an uncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist: binding and patch clamp studies. J Neural Transm Gen Sect 102:237–246
Labout JJ, Thijssen CT, Keijser GG, Hespe W (1982) Difference between single and multiple dose pharmacokinetics of orphenadrine hydrochloride in man. Eur J Clin Pharmacol 21:343–350
Lees A (2005) Alternatives to levodopa in the initial treatment of early Parkinson’s disease. Drugs Aging 22:731–740
Luzza F, Raffa S, Saporito F, Oreto G (2006) Torsades de pointes in congenital long QT syndrome following low-dose orphenadrine. Int J Clin Pract 60:606–608
Madeja M, Musshoff U, Speckmann EJ (1997) Follicular tissues reduce drug effects on ion channels in oocytes of Xenopus laevis. Eur J Neurosci 9:599–604
McDonald TV, Yu Z, Ming Z, Palma E, Meyers MB, Wang KW, Goldstein SA, Fishman GI (1997) A minK-HERG complex regulates the cardiac potassium current I(Kr). Nature 388:289–292
Mitcheson JS, Chen J, Lin M, Culberson C, Sanguinetti MC (2000) A structural basis for drug-induced long QT syndrome. Proc Natl Acad Sci USA 97:12329–12333
Redfern WS, Carlsson L, Davis AS, Lynch WG, MacKenzie I, Palethorpe S, Siegl PKS, Strang I, Sullivan AT, Wallis R, Camm AJ, Hammond TG (2003) Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc Res 58:32–45
Sánchez-Chapula JA, Navarro-Polanco RA, Sanguinetti MC (2004) Block of wild-type and inactivation-deficient human ether-a-go-go-related gene K+ channels by halofantrine. Naunyn Schmiedeberg’s Arch Pharmacol 370:484–491
Sanguinetti MC, Jiang C, Curran ME, Keating MT (1995) A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell 81:299–307
Scholz EP, Zitron E, Kiesecker C, Lueck S, Kathöfer S, Thomas D, Weretka S, Peth S, Kreye VAW, Schoels W, Katus HA, Kiehn J, Karle CA (2003) Drug binding to aromatic residues in the HERG channel pore cavity as possible explanation for acquired Long QT syndrome by antiparkinsonian drug budipine. Naunyn Schmiedeberg’s Arch Pharmacol 368:404–414
Scholz EP, Zitron E, Kiesecker C, Lück S, Thomas D, Kathöfer S, Kreye VAW, Katus HA, Kiehn J, Schoels W, Karle CA (2005) Inhibition of cardiac HERG channels by grapefruit flavonoid naringenin: implications for the influence of dietary compounds on cardiac repolarisation. Naunyn Schmiedeberg’s Arch Pharmacol 371:516–525
Sesti F, Abbott GW, Wei J, Murray KT, Saksena S, Schwartz PJ, Priori SG, Roden DM, George ALJ, Goldstein SA (2000) A common polymorphism associated with antibiotic-induced cardiac arrhythmia. Proc Natl Acad Sci USA 97:10613–10618
Thomas D, Gut B, Wendt-Nordahl G, Kiehn J (2002) The antidepressant drug fluoxetine is an inhibitor of human ether-a-go-go-related gene (HERG) potassium channels. J Pharmacol Exp Ther 300:543–548
Thomas D, Wu K, Kathöfer S, Katus HA, Schoels W, Kiehn J, Karle CA (2003) The antipsychotic drug chlorpromazine inhibits HERG potassium channels. Br J Pharmacol 139:567–574
Thomas D, Hammerling BC, Wu K, Wimmer A, Ficker EK, Kirsch GE, Kochan MC, Wible BA, Scholz EP, Zitron E, Kathöfer S, Kreye VAW, Katus HA, Schoels W, Karle CA, Kiehn J (2004) Inhibition of cardiac HERG currents by the DNA topoisomerase II inhibitor amsacrine: mode of action. Br J Pharmacol 142:485–494
Trudeau MC, Warmke JW, Ganetzky B, Robertson GA (1995) HERG, a human inward rectifier in the voltage-gated potassium channel family. Science 269:92–95
Van Herreweghe I, Mertens K, Maes V, Ramet J (1999) Orphenadrine poisoning in a child: clinical and analytical data. Intensive Care Med 25:1134–1136
Acknowledgements
This work was supported by a grant of the Deutsche Forschungsgemeinschaft KA 1714/1-2 to C. A. Karle and TH1120/1-1 to D. Thomas. E. Scholz, E. Zitron, and C. Kiesecker were supported by the Young Investigator Award of the Medical Faculty Heidelberg. This work was supported in parts by grants from the Deutsche Stiftung für Herzforschung (F/10/03 to D. Thomas), and from the German Cardiac Society (Max Schaldach Research Scholarship to D. Thomas).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scholz, E.P., Konrad, F.M., Weiss, D.L. et al. Anticholinergic antiparkinson drug orphenadrine inhibits HERG channels: block attenuation by mutations of the pore residues Y652 or F656. Naunyn-Schmied Arch Pharmacol 376, 275–284 (2007). https://doi.org/10.1007/s00210-007-0202-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-007-0202-6