Abstract.
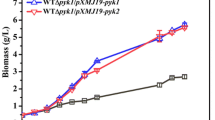
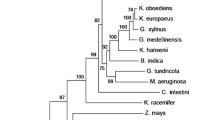
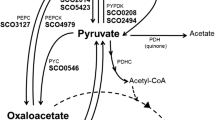
Acetobacter pasteurianus, an obligately oxidative bacterium, is the first organism shown to utilize pyruvate decarboxylase (PDC) as a central enzyme for oxidative metabolism. In plants, yeast, and other bacteria, PDC functions solely as part of the fermentative ethanol pathway. During the growth of A. pasteurianus on lactic acid, the central intermediate pyruvate is cleaved to acetaldehyde and CO2 by PDC. Acetaldehyde is subsequently oxidized to its final product, acetic acid. The presence of the PDC enzyme in A. pasteurianus was confirmed by zymograms stained for acetaldehyde production, enzyme assays using alcohol dehydrogenase as the coupling enzyme, and by cloning and characterization of the pdc operon. A. pasteurianus pdc was also expressed in recombinant Escherichia coli. The level of PDC activity was regulated in response to growth substrate, highest with lactic acid and absent with mannitol. The translated PDC sequence (548 amino acids) was most similar to that of Zymomonas mobilis, an obligately fermentative bacterium. A second operon (aldA) was also found which is transcribed divergently from pdc. This operon encodes a putative aldehyde dehydrogenase (ALD2; 357 amino acids) related to class III alcohol dehydrogenases and most similar to glutathione-dependent formaldehyde dehydrogenases from α-Proteobacteria and Anabeana azollae.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Chandra Raj, K., Ingram, L.O. & Maupin-Furlow, J.A. Pyruvate decarboxylase: a key enzyme for the oxidative metabolism of lactic acid by Acetobacter pasteurianus. Arch Microbiol 176, 443–451 (2001). https://doi.org/10.1007/s002030100348
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s002030100348