Abstract
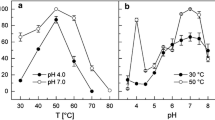
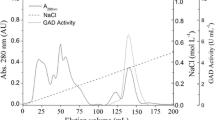
NADP+-specific glutamate dehydrogenase (EC 1.4.1.4) was purified to homogeneity from the extremely thermophilic, strictly anaerobic, sulfate-reducing archaeon Archaeoglobus fulgidus strain 7324. The native enzyme (263 kDa) is composed of subunits of mol. mass 46 kDa, suggesting a hexameric structure. The temperature optimum for enzyme activity was > 95° C. The enzyme was highly thermostable, having a half-life of 140 min at 100° C. Potassium phosphate, KCl, and NaCl enhanced the thermal stability and increased the rate of activity three- to fourfold. The N-terminal 26-amino-acid sequence showed a high degree of similarity to glutamate dehydrogenases from Pyrococcus spp. and Thermococcus spp.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 25 March 1997 / Accepted: 11 July 1997
Rights and permissions
About this article
Cite this article
Aalén, N., Steen, I., Birkeland, NK. et al. Purification and properties of an extremely thermostable NADP+-specific glutamate dehydrogenase from Archaeoglobus fulgidus. Arch Microbiol 168, 536–539 (1997). https://doi.org/10.1007/s002030050533
Issue Date:
DOI: https://doi.org/10.1007/s002030050533