Abstract
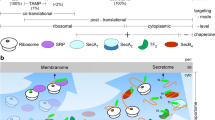
Translocation of precursor proteins across the cytoplasmic membrane in bacteria is mediated by a multi-subunit protein complex termed translocase, which consists of the integral membrane heterotrimer SecYEG and the peripheral homodimeric ATPase SecA. Preproteins are bound by the cytosolic molecular chaperone SecB and targeted in a complex with SecA to the translocation site at the cytoplasmic membrane. This interaction with SecYEG allows the SecA/preprotein complex to insert into the membrane by binding of ATP to the high affinity nucleotide binding site of SecA. At that stage, presumably recognition and proofreading of the signal sequence occurs. Hydrolysis of ATP causes the release of the preprotein in the translocation channel and drives the withdrawal of SecA from the membrane-integrated state. Hydrolysis of ATP at the low-affinity nucleotide binding site of SecA converts the protein into a compact conformational state and releases it from the membrane. In the absence of the proton motive force, SecA is able to complete the translocation stepwise by multiple nucleotide modulated cycles.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 4 August 1995 / Accepted: 9 October 1995
Rights and permissions
About this article
Cite this article
den Blaauwen, T., Driessen, A. Sec-dependent preprotein translocation in bacteria. Arch Microbiol 165, 1–8 (1996). https://doi.org/10.1007/s002030050289
Issue Date:
DOI: https://doi.org/10.1007/s002030050289