Abstract.
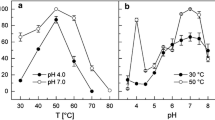
The pyruvate carboxylase (PYC) of the hyperthermophilic, strictly hydrogenotrophic, autotrophic and marine methanarchaeon Methanococcus jannaschii was purified to homogeneity. Optimal activity was at pH 8.5, ≥80 °C, and a KCl concentration of 0.175 M. This enzyme is the most thermophilic PYC so far studied. Unlike the Methanobacterium thermoautotrophicum enzyme, Mc. jannaschii PYC was expressed in cells grown without an external source of biotin and in the purified form was stable during storage at 4, –20 and –80 °C. However, it was rapidly inactivated at 80 °C. The enzyme was insensitive to aspartate and glutamate, mildly inhibited by α-ketoglutarate, and was strongly inhibited by ATP and ADP (apparent K m for ATP, 0.374±0.039 mM; apparent K i for ATP, 5.34±2.14 mM; K i for ADP, 0.89±0.18 mM). It was also strongly inhibited when the Mg2+ concentration in the assay exceeded that of ATP. Thus, this stable PYC could serve as a model for mechanistic studies on archaeal PYCs. It was apparently an α4β4-type PYC composed of a non-biotinylated 55.5-kDa subunit (PYCA) and a 64.2-kDa biotinylated subunit (PYCB). The determined NH2-terminal sequences for these subunits provided additional support for our earlier proposal to rename the ORFs MJ1229 and MJ1231 in the NCBI Mc. jannaschii genome sequence database as PYCA and PYCB, respectively; even very recently, these have been misidentified as a subunit of acetyl-CoA carbxoylase (AccC) and the α-subunit of ion-pumping oxaloacetate decarboxylase (OADα), respectively.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Mukhopadhyay, B., Patel, V. & Wolfe, R. A stable archaeal pyruvate carboxylase from the hyperthermophile Methanococcus jannaschii. Arch Microbiol 174, 406–414 (2000). https://doi.org/10.1007/s002030000225
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s002030000225