Abstract.
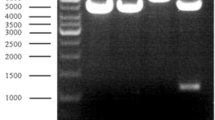
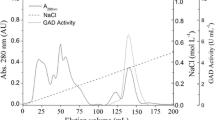
Alanine dehydrogenase [l-alanine:NAD+ oxidoreductase (deaminating), EC 1.4.1.4.] catalyses the reversible oxidative deamination of l-alanine to pyruvate and, in the anaerobic bacterium Bilophila wadsworthia RZATAU, it is involved in the degradation of taurine (2-aminoethanesulfonate). The enzyme regenerates the amino-group acceptor pyruvate, which is consumed during the transamination of taurine and liberates ammonia, which is one of the degradation end products. Alanine dehydrogenase seems to be induced during growth with taurine. The enzyme was purified about 24-fold to apparent homogeneity in a three-step purification. SDS-PAGE revealed a single protein band with a molecular mass of 42 kDa. The apparent molecular mass of the native enzyme was 273 kDa, as determined by gel filtration chromatography, suggesting a homo-hexameric structure. The N-terminal amino acid sequence was determined. The pH optimum was pH 9.0 for reductive amination of pyruvate and pH 9.0–11.5 for oxidative deamination of alanine. The apparent K m values for alanine, NAD+, pyruvate, ammonia and NADH were 1.6, 0.15, 1.1, 31 and 0.04 mM, respectively. The alanine dehydrogenase gene was sequenced. The deduced amino acid sequence corresponded to a size of 39.9 kDa and was very similar to that of the alanine dehydrogenase from Bacillus subtilis.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Laue, H., Cook, A. Purification, properties and primary structure of alanine dehydrogenase involved in taurine metabolism in the anaerobe Bilophila wadsworthia. Arch Microbiol 174, 162–167 (2000). https://doi.org/10.1007/s002030000190
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s002030000190