Abstract
Kinases can be grouped into 20 families which play a vital role as a regulator of neoplasia, metastasis, and cytokine suppression. Human genome sequencing has discovered more than 500 kinases. Mutations of the kinase itself or the pathway regulated by kinases leads to the progression of diseases such as Alzheimer’s, viral infections, and cancers. Cancer chemotherapy has made significant leaps in recent years. The utilization of chemotherapeutic agents for treating cancers has become difficult due to their unpredictable nature and their toxicity toward the host cells. Therefore, targeted therapy as a therapeutic option against cancer-specific cells and toward the signaling pathways is a valuable avenue of research. SARS-CoV-2 is a member of the Betacoronavirus genus that is responsible for causing the COVID pandemic. Kinase family provides a valuable source of biological targets against cancers and for recent COVID infections. Kinases such as tyrosine kinases, Rho kinase, Bruton tyrosine kinase, ABL kinases, and NAK kinases play an important role in the modulation of signaling pathways involved in both cancers and viral infections such as COVID. These kinase inhibitors consist of multiple protein targets such as the viral replication machinery and specific molecules targeting signaling pathways for cancer. Thus, kinase inhibitors can be used for their anti-inflammatory, anti-fibrotic activity along with cytokine suppression in cases of COVID. The main goal of this review is to focus on the pharmacology of kinase inhibitors for cancer and COVID, as well as ideas for future development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Protein tyrosine kinases (PTKs) occur in multicellular organisms’ evolution. PTKs are not found in yeasts like Saccharomyces cerevisiae or Candida albicans, but they are found in nematodes, Drosophila, and Dictyostelium. PTKs are specialized in cell-to-cell communication identified over the last twenty-five years. PTKs are comprised of the immune system, angiogenesis, cell proliferation, metabolism, and embryonic development. Due to their important roles in the multicellular organism’s function, PTK dysfunction may lead to illness (Greene et al. 2015). PTKs are usually more active in illnesses because of different mutations in the PTK or its activation systems, resulting in increased and sometimes chronic stimulation (Lemmon and Schlessinger 2010). The PTK may be absent or have reduced function in some of the illnesses condition like diabetes. Non-receptor PTKs do not contain a domain that faces the outside of the cell, while receptor PTKs obtain signals from the outer cell and transfer them within the cell. A glycoprotein called RTK (receptor tyrosine kinase) spans the cell membrane with its extracellular domains facing outward, connecting with GFs (growth factors), activating the catalytic intracellular domains, and sends signal.
Ligand binding is essential for RTK activation as it creates active oligomers or dimers by stabilizing connections between oligomeric or monomeric receptors. Activities of RTK are primarily based on the signaling molecules phosphorylation and the transcription factors activation, which mediate the target genes expression in response to ligands. Receptor recycling and internalization in physiological as well as pathological processes may also control these activity. Many biochemical processes and molecular mediators are required in the signaling pathways of receptor tyrosine kinases (RTKs), resulting in complicated signaling networks such as in EGF receptor including 322 signaling molecules and 211 biochemical reactions (Oda et al. 2005). In reality, efforts to model this network appear to be extremely difficult owing to the need for increasingly complicated spatial and stochastic characteristics. In this approach, a limited number of intermediaries are influenced by the set of RTKs, like Ca2 + signaling (core processes), “Phosphoinositide 3-Kinase (Pi3K)” and “Mitogen-Activated Protein Kinases (MAPK)” (Schlessinger 2000). RTKs primarily activate the mTOR/Pi3K/protein kinase B (AKT), “signal transducer and activator of transcription (STAT) protein” family/JAK, MAPK/RAS (rat sarcoma), and Ca2 + /calmodulin-dependent protein kinase-PKC (CaMK-PKC)/PLC (phospholipase C) pathways, and multicellular mechanisms’ downstream effectors throughout cancer progression and in the pathogenesis of COVID. (Cooke et al. 2017; Schlessinger 2000; Yu and Cui 2016). The mTOR/AKT/Pi3K pathway is critical for sustaining pluripotency because it regulates metabolism, cell growth, and survival. The RAS/MAPK system controls metabolism, cell cycle, migration, differentiation as well as proliferation. STAT regulates signaling triggered with FGF (“Fibroblast Growth Factor”), the EGF (“Epidermal Growth Factor”), the PDGF (“Platelet-Derived Growth Factor”), or lymphokines and is thus included in various cellular changes. Finally, survival, cell motility, and proliferation are all regulated by PKC. Further the pathways of MAPK/RAS and Pi3K/AKT interact in different nodes, such as ERK (“Extracellular Signal-Regulated Kinases”), and control themselves via negative and positive responses based on the cellular environment. STAT pathway molecules may also activate these two pathways, and finally, activation of MAPK has been demonstrated by PKC (Aksamitiene et al. 2012; Liang et al. 2019; Liebmann 2001).
The intracellular signaling produced by RTKs regulates several neoplastic features (migration, proliferation, metabolic abnormality, etc.), and many important kinases in these pathways (STAT, JAK, RAS, Pi3K, etc.) are often altered in cancer. As cell signaling molecular deregulation is required for the development of cancer hallmarks, categorizing cancer based on abnormalities is complementary to histological categorization (Offin et al. 2018). The therapeutic approach is no longer based on histology but solely on the molecular abnormalities in some clinical studies termed as “agnostics.” These clinical studies are presently examining BRAF, HER2 (“Human Epidermal Growth Factor Receptor-2”) inhibitors, and mTOR/AKT/Pi3K/ or RAF (“Rapidly Accelerated Fibrosarcoma”)/MEPK) pathways on mutated cancers. This group of RTKs includes ∼60 well-known proteins, like EGFR (EGF receptor), PDGFR (PDGF receptor), InsR, VEGFRs (“Vascular Endothelial Growth Factor Receptors”), and more. Non-receptor PTKs, like Src group, Jak, and Abl group, are stimulated with upstream signaling molecules, including RTKs, immune system receptors as well as G protein-coupled receptors.
The Darwinian selection was suggested for cancer cell proliferation to maintain proliferation under adverse circumstances and during TME (“Tumor Microenvironment”) imposed alterations. A class of drugs that target intracellular signaling has been created to go after the molecular pathways’ hubs. RTKIs (“Receptor Tyrosine Kinase Inhibitors”) are a broad class of targeted medicines that have seen extensive clinical usage since 2001 with remarkable results (Fouad and Aanei 2017). Cell proliferation and angiogenesis are two common processes that are inhibited by kinase inhibitors that target the kinase active site and block the phosphorylation of intracellular targets. The FDA (“Food and Drug Administration”) has authorized 43 RTK inhibitors for oncological use as of August 2019 (Yamaoka et al. 2018). They may be differentiated from irreversible ones by the fact that they bind covalently with or near the binding site of ATP (“Adenosine Triphosphate”). Non-covalent inhibitors include several ATP-competitive inhibitors that connect to type I inhibitors (active conformations). The ATP-binding sites are usually maintained, and selectiveness may be accomplished by concentrating on less well-preserved residues, such as those near the hinge. Type II inhibitors attach to an area around the ATP site on inactive kinases and keep them in an inactive state. Non-selective inhibitors are the most prevalent type among them (Crisci et al. 2019; Fabbro et al. 2015; Roskoski 2019). An allosteric inhibitor blocks kinases by attaching to an allosteric site other than the one that is connected to ATP. These types of inhibitors (type III) are very selective in nature. Inhibitors of substrate-binding sites that are reversible, such as type IV RTKIs, are currently being developed. These type IV inhibitors are a therapeutic focus for metastatic cancers. Type V inhibitors also known as fibroblast growth family receptors have the benefit of being powerful while having fewer off-target effects than non-covalently bound kinase inhibitors (Bhullar et al. 2018).
Types of PTK inhibitors
Tyrphostins
PTK inhibitors were first developed in the early 1980s when natural substances like lavendustin A, genistein, erbstatin, and quercetin were discovered to inhibit the activities of PTKs like EGFR and pp60Src. Despite their lack of selectivity and average efficacy, these natural compounds were utilized as an initial point for the creation of synthetic PTK inhibitors that were more powerful and more selective (tyrphostins) (Gazit et al. 1989; Shechter et al. 1989). The action of itaconic acid and the findings of Umezawa et al. 1986 on erbstatin prompted the first systematic production of many tyrphostins in the benzene malononitrile family. Itaconic acid as well as erbstatin’s structure acted as a template for a huge range of new substances, several of which showed good PTK inhibition but no important inhibition of Thr/Ser kinases. This first group of tyrphostins demonstrated that a series of substances may be synthesized that block a specific PTK with little toxicity in cells and in vivo (Kovalenko et al. 1994; Osherov et al. 1993).
Chronic myelogenous leukemia (CML), Bcr-Abl kinase, and its inhibitors
CML is caused by PTK Bcr-Abl kinase. During the first three to five years of the disease’s chronic phase, CML cells are completely based on the Bcr-Abl kinase activity for survival. This proposed that preventing this enzyme would cause the removal of the unhealthy cells from the body of the patient. In 1992, researchers discovered the first powerful and highly selective Bcr-Abl kinase inhibitors. AG 957 and their derivatives, a class of inhibitors, compete with the substrate but not with ATP. AG 957 causes apoptosis in CML cells and works in conjunction with CH 11, an anti-Fas antibody that promotes apoptosis (Anafi et al. 1993). The AG 957 analogue adaphostin is being tested in the clinic. K562 (CML) cells undergo significant apoptosis when treated with AG 1112 or AG 1318, two members of the Bcr-Abl selective agent family that are ATP-competitive. Novartis and Druker team noted in 1996 on a Bcr-Abl inhibitor, “CGP 57148,” subsequently called STI-571/Gleevec/Glivec/imatinib mesylate (Carlo-Stella et al. 1999). STI-571 selectively inhibits Bcr-Abl by interacting with the amino acids that make up the PTK’s ATP-binding cleft in its inactive form. Individuals in the chronic phase of the illness show long-lasting responses in clinical trials, while those with more advanced diseases experience recurrence (Anafi et al. 1993).
The fact that STI-571 is well tolerated and has few adverse effects is very encouraging. This came as a surprise at first, since STI-571 is known to inhibit c-Abl, c-Kit as well as PDGFR. This family of kinases is essential in healthy cells, and therefore, removing their genes has serious consequences. The most probable reason is that healthy cells that employ PDGFR, c-Kit, or c-Abl can survive even when nearly 90 percent of these targets are inhibited as they can make use of the other mechanism that all normal cells acquire. On the other side, CML cells are based on Bcr-Abl for their survival and thus die when it is inhibited. “STI-571” is also successful in the treatment of GIST (“Gastrointestinal Stromal Tumor”), particularly in individuals with activating c-Kit mutations in exon 11 (Carlo-Stella et al. 1999).
EGFR kinase inhibitors
More than 25 EGFR inhibitors were reported in the last ten years, many of which are in different phases of preclinical and clinical research. (Abourehab MAS et al. 2021).
Reversible inhibitors
First, AG1478 was found to be an effective EGFFR kinase inhibitor. Later, gefitinib, an anilinoquinazoline that was more soluble, was created. However, the captisol-based AG1478 is still in clinical development and has not yet made it to a clinical setting for testing. Gefitinib is a drug that reduces the kinase activity of the EGFR and is taken orally. Her-2 is ∼100 times less effectively blocked by gefitinib, which binds to the ATP site. Erlotinib is a substance which is comparable to gefitinib (Chandra 2006). NSCLC (“Non-Small Cell Lung Cancer”) is treated with gefitinib since 2002; however, it only works in a limited proportion of individuals with EGFR mutations that activate the kinase domain. Erlotinib had effects that were comparable to those of Gefitinib. Individuals who react to these drugs have specific EGFR kinase domain activating mutations, and tumor survival appears to be dependent on the activated version of the EGFR in these patients. EGFR overexpression is a characteristic in all other instances, but tumor survival does not seem to be dependent on EGFR activation, which explains the lack of effectiveness of EGFR kinase inhibitors (Anafi et al. 1993; Carlo-Stella et al. 1999). It is impossible to determine whether the failure of erlotinib and gefitinib is owing to a lack of an EGFR survival function or insufficient long-run receptor occupancy without precise measures of EGFR phosphorylation inpatient cancers. Researchers’ discovery that an inhibitor of irreversible EGFR kinase is more effective against tumor validates the latter theory (Blanke and Corless 2005). There are currently no novel reversible EGFR kinase inhibitors in development because of the poor results of gefitinib and erlotinib. According to recent research, 10–20% of patients with EGFR-overexpressed glioblastoma multiforme respond well to an EGFR kinase inhibitor. There appears to be a connection between their reactions and PTEN and mutant EGFR expression. The results indicate that EGFR kinase inhibitors would enter clinical trials in situations when receptors have survival functions on their own or when inhibitors are used along with other signaling pathways agents to induce apoptosis in cancer cells particularly (Ward et al. 1994).
Irreversible inhibitors
All of the reversible EGFR kinase inhibitors currently on the market are ATP competitive. To exert anticancer action, reversible ATP inhibitors must contend with large levels of endogenous ATP inside the cell over time. Furthermore, their fast clearance from plasma requires sustained delivery. PET (“Positron Emission Tomography”) investigations on tumor-bearing animals scanned with “fluorine-18-labeled” reversible EGFR inhibitors showed that reversible inhibitors wash out of the tumor region quickly (Osherov and Levitzki 1994). Furthermore, imaging mice with EGFR-overexpressing tumors using fluorine-18-labeled gefitinib revealed no evidence of long-term or significant absorption by the tumor. The development of irreversible EGFR kinase inhibitors based on 4-(phenylamino) quinazoline, as well as quinoline core structures, was a significant attempt to improve therapeutic efficiency (Wakeling 2002). Most of the work was done by connecting a functional group of the Michael acceptor to the quinoline or anilinoquinazoline basic structure, which allowed the inhibitor to establish covalent bonding via electrophilic assault on a cysteine residue inside the binding pocket. This approach was based on the discovery that the kinase-specific cysteines Cys-751 in Her-2, as well as Cys-773 in EGFR, occupy the only positions in the binding pocket of ATP (Kimura et al. 2006). The 6th position of the quinazoline, as well as quinoline rings, produced the greatest results in the synthesis of a variety of irreversible EGFR as well as Her-2 inhibitors with various functional groups of Michael acceptor added at the 6- and 7-positions (Paez et al. 2004). An effective and long-lasting EGFR and Her-2 inhibitor has been discovered in “PD168393,” which has the functional group of acrylamido at the 6-position (Denny 2001; Discafani et al. 1999; Tsou et al. 2001; Vincent et al. 2000). CI-1033, EKB-569, and HKI 272 are all irreversible inhibitors presently in clinical trials. It is interesting to note that an irreversible inhibitor of EGFR is efficient against gefitinib-resistant EGFR kinase mutants, indicating the requirement for long-term receptor occupancy with little toxicity (Rabindran et al. 2004).
VEGF receptor tyrosine kinase inhibitors
Endothelial cell survival, proliferation as well as migration were implicated in tumor angiogenesis, with VEGFRs serving as key mediators in each of these processes. There are a variety of roles for VEGFR-2 in tumor angiogenesis, with endothelial cells being the most abundant source. As a result, this receptor is now a popular therapeutic target for a wide range of malignancies. Fighting cancer by cutting off the tumor’s blood supply is a major approach (Smaill et al. 2000). Numerous animal studies and preclinical trials have supported this notion. Angiogenesis inhibitors were first shown to be useful when it was discovered that some tyrphostins may suppress KDR/VEGFR-2 and therefore angiogenesis in vivo. Several VEGFR kinase inhibitors were created during the following several years, and a few made it into clinical trials (Smaill et al. 1999).
Many small organic chemical families were created as possible VEGFR tyrosine kinase inhibitors based on the VEGFR-2 kinase domain crystal structure. 4-anilinoquinazolines and 3-substituted indolinones are two examples of these compounds (Shaul et al. 2004). SU 11248, which targets FLT3, Kit, PDGFR, and VEGFR, appears to outperform its predecessors in the group of 3-substituted indolinone (Mishani et al. 2005). This is likely due to the compound’s multitargeted nature, which targets a variety of signaling RTKs associated with tumor blood supply at the same time. SU 11248 showed wide and strong anticancer efficacy in mouse xenograft models. Because the anticancer action is coupled with anti-angiogenic activity, the substance is highly efficient in prostate cancer xenograft models where angiogenesis and PDGFR have an important role. Patients with AML (“Acute Myeloid Leukemia”) who received SU 11248 showed lower levels of Flt3 in the blood. The Kit kinase is essential for the survival of several GIST cases. Since SU 11248’s capability to inhibit Kit, particularly STI-571-resistant Kit, performed so well in STI-571-resistant GIST, clinical studies for the drug have been accelerated. The anti-angiogenic potential of SU 11248 has yet to be established (Dvorak 2003; Robinson et al. 2000; Strawn et al. 1996).
BAY 43–9006 is another potential inhibitor of VEGFR-2 kinase. Firstly, “BAY 43-9006” was identified as an orally accessible B-Raf (c-Raf) kinase inhibitor, raising hopes that it might be useful in the treatment of metastatic melanoma, which often has an activating B-Raf mutation. It also inhibits c-Kit, p38, PDGFR, FLT-3, VEGFR-3, and VEGFR-2 (Checkley et al. 2003; Hennequin et al. 2002; McTigue et al. 1999). The agent was soon shown to be ineffective against melanoma, but beneficial against renal cancer in the clinical setting. Anti-angiogenic inhibition of VEGFR-2 may account for some of BAY 43-9006’s therapeutic usefulness (McCarty et al. 2004; Sun et al. 1998).
RTKI in cancer treatment
In precision oncology, RTKIs (“Receptor Tyrosine Kinase Inhibitors”) have a significant role although the expansion of resistance mechanisms limits their efficacy. This section discusses the development of RTKIs in NSCLC and other cancers, as well as resistance mechanisms.
Development of RTKI Use in NSCLC
Clinical studies using the first generation of EGFR reversible ATP-binding site (erlotinib and gefitinib in cancer patients) inhibitors revealed the usage of RTKIs in NSCLC. Patient survival was increased by 50 percent (OS (“Overall Survival”) of 30.5 months vs. 23.6 months) when these therapies were used instead of chemotherapy (Fong et al. 1999). The RTKIs second generation consists of irreversible inhibitors (dacomitinib and afatinib) that have a higher affinity for the kinase domain of EGFR and inhibit various groups of the HER family, including the EGFR. Afatinib doubles existence evaluated with chemotherapy (11.1 months vs. 6.9 months for mPFS (“median progression-free survival”) (Laird et al. 2000). Clinical trials exhibited that dacomitinib provided a longer survival time than gefitinib (mOS (“median OS”) of 34.1 months versus 26.8 months and mPFS of 14.7 months vs. 9.2 months). Dacomitinib and afatinib are more likely to cause severe side effects, such as skin ulceration or irritation, as well as gastrointestinal toxicity (vomiting, diarrhea, constipation, and severe nausea) (O’Farrell et al. 2003).
Secondary EGFR kinase domain mutations are the most frequent mechanism of treatment resistance to these agents. Consequently, the researcher developed a third-generation EGFR-RTKI, osimertinib, with improved affinity for mutated receptors. Osimertinib’s PFS was considerably longer (18.9 months vs. 10.2 months), and it had a better tolerability profile than the first-generation EGFR RTKI (Chakraborty et al. 2019). The ESMO (“European Society of Medical Oncology”) now recommends gefitinib, erlotinib, and osimertinib or afatinib as first-line therapy for NSCLCs including an EGFR-activating mutation. NSCLC patients may consider a combination of gefitinib and chemotherapy as a first-line therapy option. Other treatments, like erlotinib with ramucirumab or bevacizumab (anti-angiogenic antibodies that target the VEGFR), are being studied but are not advised as yet. The combination of VEGFR and EGFR inhibitors seems to be synergistic due to interaction in its signaling pathways and anti-angiogenic therapy-induced vascular normalization, which may enhance RTKI intra-tumor concentration. Osimertinib is the second-line therapy for patients having T790M mutation in exon 20 after systemic progression. Chemotherapy on a platinum basis with or without bevacizumab or immunotherapy is recommended in the lack of this mutation (Sequist et al. 2013). Numerous early clinical trials explore novel treatment combinations. For instance, the first generation of EGFR RTKI (gefitinib) in conjunction with osimertinib is being studied as first-line therapy in certain current clinical studies. Moreover, as a consequence of encouraging preclinical findings indicating synergistic activity (chidamide inhibits activation in pathways like Pi3K/AKT and RAS/MAPK), the HDAC (“Histone Deacetylase”) inhibitor chidamide is being investigated in conjunction with various EGFR RTKIs (Wu et al. 2017). Further examinations are assessing the effectiveness of new third generation of EGFR RTKIs (avitinib, rociletinib, nazartinib), non-selective RTKIs activating numerous RTKs, such as VEGF and EGF (momelotinib, anlotinib, sorafenib), or RTKIs that target the c-MET HGF (tepotinib, capmatinib), JAK2 (pacritinib, momelotinib), and AXL receptor tyrosine kinase (gilteritinib). These investigations must provide good findings, but it is important to keep in mind that most RTKI combination methods have failed in human trials in recent years due to a lack of efficacy or tolerability (Morgillo et al. 2016).
Current indications of RTKIs in other kinds of cancer
NSCLC having mutated EGFR may be used as an example to illustrate the development of the RTKIs that revealed limited effectiveness over the last 20 years. CML, which may be “cured” with one or more RTKIs, is treated in the same way as all other types of cancer. The ESMO recommends anti-angiogenic RTKIs like pazopanib or sunitinib as first-line treatment for individuals with a good prognosis for RCC (“Renal Cell Carcinoma”). The 2nd line therapy in the event of recurrence following RTKI is either immunotherapy or still another RTKI (axitinib or cabozantinib). The second-line efficacy is characterized by dual inhibitory action on MET and VEGFR2 or MAPK, which are active in tumor cells resistant to 1st line RTKIs (Hochmair et al. 2019).
Owing to trastuzumab’s significant toxicity, lapatinib may be utilized as a 1st line therapy in individuals with metastatic HER + BRCA in addition to that drug. In 2nd line treatment, lapatinib appears to be less successful than the antibody–drug combination trastuzumab emtansine, T-DM1 (Planchard et al. 2019). Multitarget kinase inhibitors are most often used for systemic treatment of hepatocellular cancer. In the 1st line therapy, lenvatinib (targeting PDGFRi, FGFRi, VEGFRi) or sorafenib (PDGFRi, VEGFRi) is suggested, while immunotherapy or cabozantinib (RETi, GAS6 (“growth arrest-specific 6”)i), regorafenib (PDGFRi, FGFRi, and VEGFRi), was utilized in 2nd line. In “metastatic colorectal cancer,” “regorafenib,” a multitarget kinase inhibitor (PDGFRi, FGFRi, EGFRi), is the 1st RTKI to show a small survival advantage (6.4 vs. 5 months) in patients with metastatic colorectal cancer when antibodies targeting VEGFR and EGFR failed and chemotherapy were administered. Imatinib (BCR (“Breakpoint Cluster Region”)-ABL inhibitor) transformed the prognosis of gastrointestinal stromal cancers exhibiting the KIT (CD117), RTK, and mOS of more than five years and is now the first-line treatment. Regorafenib and sunitinib may be administered after a recurrence (Pennell and Lynch 2009).
First-line treatment for differentiated thyroid tumors resistant to radiation treatment includes lenvatinib (VEGFRi) and sorafenib (SOR), both of which have been shown to increase PFS (5.8 months with placebo vs 10.8 months with sorafenib, mPFS 3.6 months with placebo vs 18.3 months with lenvatinib). Cabozantinib, as well as vandetanib (RETi, EGFRi), is 1st line systemic therapies for progressing metastatic medullar thyroid cancer (PFS is 19.3 months with placebo vs. 30.5 months with vandetanib, and 4.0 months with placebo vs 11.2 months with cabozantinib) (Zhang et al. 2019). First-line therapy for unresectable stage “III/IV BRAF V600” mutation melanoma is BRAF RTKI (dabrafenib, encorafenib, vemurafenib) coupled with MEK RTKI (binimetinib, trametinib, cobimetinib) with mPFS of one year. Immunotherapy is the only therapeutic option in the second line.
Finally, RTKIs have had the greatest success in treating CML, with an 83.3 percent survival rate for individuals treated with imatinib after ten years. The CML exception may have evolved for reasons that may be traced back to evolution. There is no allopatric speciation in CML because the cell population is homogeneous. During the chronic phase of the illness, only one main oncogene is existing in the whole population, and clonal alterations occur systematically and gradually (Yang and Tam 2018). In comparison with the dynamic and unpredictable development of solid tumors, CML develops in a clonal and progressive manner. Other reasons for resistance have been discovered in preclinical investigations, like “compensatory hyperactivation of anti-apoptotic pathways” (CRS), and efflux proteins (hOCT1 (“Human Organic Cation Transporter 1”) or P-glycoprotein) are involved. First-generation RTKIs (imatinib) or second-generation RTKIs (bosutinib, nilotinib, and dasatinib) are indicated for low-risk chronic CML, while only second-generation RTKIs are suggested for CML in the high-risk chronic stage, and ponatinib, as well as another third-generation RTKIs, is kept for the second-line therapy (Escudier et al. 2019) (Table 1).
Mechanisms of resistance to RTKIs
Usually, RTKI resistance may be intrinsic (“primary”), when the tumor does not respond to therapy or obtained (“secondary”) when resistance develops after an initial reaction to RTKI therapy, and a gradual choice of resistant cancer cells. The majority of RTKI resistance systems may be traced back to mutations in the RTK gene. It has been discovered that certain mutations in critical residues (such as a gatekeeper residue) in the catalytic domains of these enzymes prohibit RTKI from binding to the ATP-pocket of RTK via steric hindrance. Clinical trials were shown that around 50 distinct BCR-ABL mutations are liable for imatinib resistance, and participation of other intracellular signaling components, like the FOXO1 (“Forkhead Box Protein O1”), AXL, the NF-κB (“Nuclear Factor-Kappa B”), STAT3, and β-catenin in RTKI resistance. In GIS cancers expressing KIT Genomic study of cancers found mutations in EGFR and BCR-ABL gatekeeper residues, or activating mutation in BRAF, or IGF1R (“insulin-Like Growth Factor 1 Receptor”) extensions were noted in imatinib resistance. Other pathways, like KIT mutations in the kinase domain’s ATP-binding pocket or the kinase activation loop, have been studied in preclinical research (Cardoso et al. 2018; Miyazaki et al. 2016; Yu et al. 2016).
Activating a kinase parallel or downstream to the intended signaling path, or on a parallel pathway, is another method to avoid the effects of RTKI. This is the pathway of RTKI resistance most often reported. As a result of compensating signaling pathways arising when the primary one is inhibited, as well as naturally appearing cross talk and linkages between various pathways. For example, variations in pathways are frequent with EGFR activation or PTEN (“Phosphatase and Tensin Homolog”) loss but include alternate activation of VEGF, STAT3, and mTOR/AKT using RTKI-induced interleukins IL8 and IL6 autocrine secretions, and resistance (Vogel et al. 2019). Among NSCLC patients, the KRAS mutation has been shown to confer anti-EGFR RTKIs resistance in both clinical and preclinical trials. Therefore, HCC (“Hepatocellular Carcinoma”) resistance to sorafenib is related to the tumor’s over-activation of the EGFR pathway. Autophagy and Pi3K/AKT have a role in HCC’s therapeutic resistance since they both promote cell survival. Moreover, the hypoxia produced by sorafenib therapy also activates MAPK/ERK (“Extracellular Signal-Regulated Kinases”) and STAT/JAK and upregulates HIF-2α (“Hypoxia-Inducible Factor 2-Alpha”), which activates TGF-α (“Transforming Growth Factor Alpha”) and EGFR. Mutations in the RET mutation provide resistance to RTKIs in thyroid cancer. EGFR phosphorylation, PTEN loss, or activating Pi3K-AKT, NRAS, or MAPK mutations are among the common resistance systems to BRAF inhibitors confirmed on patients’ tumors (Blanke et al. 2008; Grothey et al. 2013).
Resistance to RTKIs may develop as a result of phenotypic change. The common instances are the EMT (“Epithelial–Mesenchymal Transition”) in which cancer cells lose their epithelial features, like polarity and cell–cell adhesion, in favor of mesenchymal features and of the development of an invasive phenotype. Therefore, in the cases of NSCLC (non-small cell lung cancer), translational research has been focused on EMT activation signaling pathways (Hedgehog and AXL). The histology change of pulmonary adenocarcinoma in small cell lung cancer is another uncommon alteration that contributes to NSCLC resistance to RTKIs. These mechanisms of resistance seem to be class independent (Casali et al. 2018).
The choice of cancer cells that express efflux pumps capable of transporting medicines is an important resistance strategy. For instance, in RCC, preclinical studies revealed that sunitinib and sorafenib may be sequestrated from the lysosome by ABC (“ATP-binding cassette”) transporter and P-glycoprotein (Brose et al. 2014).
RTKI combinations
Primary and secondary therapeutic resistance may be prevented by using a mixture of RTKIs, according to Pottier, C et al. (2020). Using a technique known as vertical route inhibition, the first approach seeks to block the same signaling pathway twice. It involves either reducing the severity of an RTK mutation or activating effectors farther down the pathway. In a clinical study including NSCLC patients cured with first- and third-generation anti-EGFR medicines, the decision to block the same target in two distinct methods was put to the test (Roberts et al. 2007). However, alternative combinations are still being investigated in clinical trials, including those that target an RTK and their downstream effectors, such as the combined BRAF-MEK inhibition suggested in melanoma patients with advanced disease. A single RTKI may have a twofold inhibitory effect or two selective RTKIs for the same target, which can be used to create double inhibitions. This selective dual CRAF/BRAF along with MEK inhibitor was studied in phase 1 dosage-escalation clinical trial as an example (Alexander and Wang 2015).
The development of a novel technique known as horizontal inhibition is underway. A cross talk inhibition is used to prevent a secondary route from becoming overactive in response to the inhibition of the primary pathway (Tolcher et al. 2018). Numerous promising preclinical results have led to the beginning of clinical study phase I for the cure of melanoma using an anti-MEK RTKI or BRAF in conjunction with an anti-Pi3K. Other phases 1 agnostic clinical studies have looked into the mixture of AKT and MEK. It is clear from all of these clinical studies that it is difficult to combine RTKIs because no one has found a way to balance off the higher toxicity with the greater chance of survival, as is common in trials including several RTKIs (Moradpour and Barghi 2019). Hence, RTK inhibitor toxicity is a key therapeutic issue. In part, their toxicity is because they have side effects that are unintended. RTKI affinity for other targets has to be better determined to choose the most targeted RTKIs. Another strategy involves employing nanoparticles or PEGylated liposomes to reduce the concentration of RTKIs in healthy tissues, although this strategy is still being studied, and its clinical effectiveness has yet to be shown (Moradpour and Barghi 2019).
Effect of the non-immune microenvironment on RTKI efficiency
In addition to targeting tumor cells, RTKIs also affect TME cell components. Treatment responses are indeed affected by a variety of factors, including chemotherapeutic agents, immunotherapy, and radiation. TME stromal cells exposed to RTKIs release hormones, GFs, or cytokines, that influence the tumor’s response to this. As a result, RTKIs that target FAK (“Focal Adhesion Kinase”), VEGFR, c-MET, and FGFR reduce the variety of fibroblasts or their activation, and as a result, their involvement in promoting the development of different cancer (Maifrede et al. 2018).
It is interesting to note that the TME can alter neoplastic cell signaling and promote RTKIs resistance. Thyroid carcinoma pericytes cause vemurafenib resistance via secretion of TSP-1 (“thrombospondin 1”) and TGFβ1 (“transforming growth factor beta-1”), which raises expression of the PKR (“protein kinase R”)-like “endoplasmic reticulum kinase” (pERK1/2), pAKT (“phosphorylated AKT”), and pSMAD3 (“phosphorylated mothers against decapentaplegic homolog 3”) levels. Likewise, stromal cells may secrete HGF (“hepatocyte growth factor”) which stimulates MAPK, activates MET, as well as mTOR/AKT/Pi3K, resulting in melanoma cells BRAFi resistance. CAFs (“Cancer-Associated Fibroblasts”), via the production of NRG1 (“neuregulin-1 beta”), a HER3 ligand, may cause resistance to RTKI treatment targeting HER2 (Ding et al. 2018). To enhance medication distribution inside the tumor, PDGFR and VEGFR inhibitors are given at low dosages. Tumor cells could spread more widely as a result of sunitinib therapy depleting pericytes and causing hypoxia (Wang et al. 2018).
However, there is a negative connection between the effectiveness of lapatinib in HER2 + BRCA as well as the elastic modulus of the “Extracellular Matrix,” which indicates that the more successful lapatinib is, the easier it is to deform the ECM (Pottier et al. 2015).
Effect of the immune microenvironment on RTKI efficiency
This also affects the immune microenvironment. For instance, imatinib, sorafenib, and dasatinib reduce T-regulator cells and enhance the response of anti-tumor T cells. Likewise, sunitinib reduces MDSCs (“Myeloid-Derived Suppressor Cells”) and M2 macrophages’ survival and proliferation, encouraging the development of an immune-competent TME that is permissive. Cabozantinib increases the anticancer innate immune response mediated by neutrophils. Increases in tumor-associated CD8 + cells and expression of melanoma antigens may be achieved with either BRAF or MEK inhibitors alone or in combination. For instance, added to their effect on cancer cells, RTKIs that target the FGFR decrease the variety of immunosuppressive MDSCs in the tumor as well as promote the senescence of CAFs (“Cancer-Associated Fibroblasts”). VEGFR1 inhibitors in adding to its anti-angiogenic effects may regulate tumor microvasculature and reduce the invasion of Treg lymphocytes, MDSCs, and certain immunosuppressive TAMs (“Tumor-Associated Macrophages”) populations (Tan et al. 2018).
Moreover, resistance to RTKIs was shown to be accompanied by the development of an immunosuppressive TME. Because BRAF tumors are resistant to RTKIs with the rise in MDSCs is also seen. Glioblastoma’s resistance to axitinib resulted in a rise in Treg cells and the development of the PD-1 inhibitory checkpoint. The development of resistance to imatinib in GISTs (“gastro-intestinal stromal tumors”) was associated with a change in TAM phenotype. Furthermore, RTKIs that target EGFRs are less effective in individuals whose tumors are heavily packed with CD8 + lymphocytes and have high concentrations of PD-L1 (“Programmed Death-Ligand 1”). Besides that, immune cells in the microenvironment are capable of acting in various ways. As a result, MDSCs recruited during therapy may generate angiogenesis-stimulating proangiogenic substances without relying on VEGF (KATOH 2016).
As a result, combining RTKIs with immunotherapies is a logical step forward. There have only been a few clinical investigations done on the topic, and the toxicity of these mixtures is typically very significant. Though, atezolizumab with sunitinib were combined in a phase-3 study for metastatic RCC, while pembrolizumab with trametinib and dabrafenib (BRAF inhibitor) was used in a phase-2 trial for BRAF-mutant melanoma patients. Both trials had promising outcomes with mild side effects. Similar findings were seen with the mixture of lenvatinib as well as pembrolizumab in an endometrial and kidney cancer phase-2 clinical study, and also with the grouping of regorafenib and nivolumab, assessed in a phase-2 clinical study in colorectal cancers or advanced gastric (Prete et al. 2018). Non-neoplastic cells’ tyrosine kinase receptors, including AXL, the TAM-produced RTK that is deemed a new class of innate immune checkpoints, may potentially become an interesting therapeutic target. In light of these elements, several early clinical trials are presently examining checkpoint and RTKI mixtures (for example, nivolumab mixed with sunitinib or pazopanib, axitinib mixed with pembrolizumab).
Synthetic lethality and receptor tyrosine kinase inhibitors
When a single therapy does not induce cell death, combining it results in synthetic lethality. The PARP (“Poly-Adenosine Diphosphate Ribose Polymerase”) inhibitor was one of the earliest synthetic lethality achievements, and it is being utilized in clinics to produce synthetic lethality in BRCA (“Breast Cancer”). Maifrede et al. recently revealed that inhibiting FLT3 (“Fms-like tyrosine kinase 3”) with an RTKI seems to downregulate critical proteins involved in DNA “double-strand break” repair in acute myeloid leukemia, like RAD51, BRCA2, and BRCA1. The combination of the FLT3 RTKI and a PARP inhibitor showed extremely promising outcomes in mouse models in this research. Other treatment classes, such as metabolic inhibitors, may benefit from this strategy. Ding et al. thus recognized that transaldolase, an enzyme in the non-oxidative “pentose phosphate pathway” to be critical to tumor cells survival treated using lapatinib in CRISPR (“Clustered Regularly Interspaced Short Palindromic Repeats”) knock-out study. Due to the enzyme inactivation and the simultaneous inhibition of HER2, NADPH (“Nicotinamide Adenine Dinucleotide Phosphate”) level was decreased, which enhanced the production of ROS (“Reactive Oxygen Species”) while decreasing the synthesis of the nucleotides as well as lipids.
Drug resistance may be linked to apoptosis regulating mechanisms. ATO (“Arsenic trioxide”) promotes tumor cell differentiation, making it an effective therapy for a rare form of leukemia termed “acute promyelocytic leukemia.” Wang et al. have shown that ATO generates a large number of apoptotic signals but fails to induce apoptosis due to the phosphorylation along with consequent inactivation of GSK3β (“glycogen synthase kinase3”), a major proapoptotic enzyme. Sorafenib, which activates GSK3β and increases the rate of apoptosis while also extending mouse tumor model life, was evaluated, and shown to be effective (Chan et al. 2017).
JAK-2 and JAK-3
During the early stages of the cytokine signaling pathway, Janus kinases (Jaks) play an important role as cytoplasmic PTKs. Oncogenic mutations increase Jak-2’s activity and create constitutive Jak-2 fusion proteins, which play a vital function in different lymphomas, leukemia, and some types of metastatic cancers like prostate and breast cancer (Chan et al. 2017). Thus, Jak-2 was discovered as a possible cancer treatment target fairly early on, but no therapeutic drug that targets this kinase was produced for clinical usage. The first Jak-2 inhibitor developed, AG 490, was used effectively in animal models to eradicate leukemia, myeloma, and lymphomas. Analogs of AG 490 were developed since then, with varying degrees of activity in vivo. The Stat3/5/Jak-2 pathway, a critical route for maintaining the oncogenic phenotype, was blocked in all cases studied by AG 490 and its analogs (Straussman et al. 2012).
JAK-3 inhibitors
JAK3 is highly expressed in “lymphoid cells” and starts IL-15, IL-13, IL-9, IL-7, IL-4, and IL-2, signaling. JAK3 is implicated in T cell proliferation as well as activation, which is important for autoimmune and leukemia diseases or transplant-related inflammatory conditions. The specific JAK3 targeting in T cells could be therapeutically effective in T cell-derived pathologic diseases, such as the rejection of the solid organ, allergy, and autoimmune diabetes. Jak-3 inhibitors were previously progressed and examined in a variety of different diseases.
Repurposing of PTK inhibitors for COVID treatment
Several PTK inhibitors such as IL-1 receptor antagonists, JAK inhibitors, Numb-associated kinases, cyclin-dependent kinases, AXL kinases, GSK-3β inhibitors, and P38 inhibitors have been found to show significant clinical improvement in the treatment of COVID.
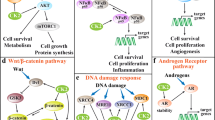
IL-1 plays a significant role in the initiation and the progression of cancerous cells also leading toward chronic inflammatory condition seen in the cases of COVID-19 patients. Tumor-associated macrophages known as TAM contribute toward growth of cancerous cells, causing angiogenesis, reducing the adaptive immunity. The cytokines which are expressed during inflammatory conditions can trigger the carcinogenic response of TAM. Patients of COVID were found to have increasing amounts of cytokines leading toward cytokine storm. Therefore, IL-1 receptor antagonists can be considered for the lessening of the effects of cytokine storm seen in cases of COVID.
Alterations in the cell cycle which is associated with proliferation of tumor cells can be caused due to the activity of cyclin-dependent kinases (CDKs). These kinases are responsible for the cell cycle progression. Various CDK inhibitors were discovered to inhibit cancer cell proliferation in clinical trials. The COVID virus was found to alter the signaling pathway of CDK which can cause increased cell cycle replication. Therefore, CDK inhibitors can help in the reduction of viral replication and also provide additive synergistic anti-viral action when used in combination with other anti-virals (Lacal and Graziani 2018). AXL receptor tyrosine kinases (AXL) belong to the TAM family, in which dysregulation of the TAM signaling can lead toward cancer progression and chronic inflammatory condition. AXL expression is high in primary tumors and metastases, and AXL serves as an entry way SARS-CoV (Lei et al. 2020). AXL inhibitors can serve as a therapeutic target to reduce the viral infective load as well as provide future clinical intervention strategies (Kralovics et al. 2005). Aberrant expression of GSK-3β can lead toward increased survival of cancerous cells, proliferation and invasion into various tissues, anticancer drug resistance, etc. Studies have indicated that the inhibitors of GSK-3β can be helpful in reducing viral transcription and replication which can be utilized in decreasing the viral load (Gargouri et al. 2021).
Numb which is an endocytic adaptor contributes toward the progression of various cancers such as hepatocellular carcinomas (HCC) and anaplastic large cell lymphomas (ALCL). Numb, a membrane-associated protein, interacts with Notch and numb-associated kinase (Nak) which was found to be indicated in tumorigenesis due to increase in levels of Numb expression. The inhibitors of the Numb family were found to be effective in controlling the levels of viral infections in COVID. P38 mitogen-activated protein kinases (MAPK) contributes to larger extent in cases of cell proliferation in colon cancers, lymphomas, etc. These targets when inhibited show significant anti-inflammatory activity which can be repurposed for COVID. The pathogenic inflammatory response seen in cases of COVID can be reduced with the help P38 MAPK inhibitors (Rankin and Giaccia 2016). The next section reviews the drugs which were repurposed for the treatment of COVID by reduction in the viral load, preventing replication of viral cells, improving innate immunity, and by reducing the effects of cytokine storm.
Role of PTK in COVID
Coronavirus disease, or COVID-19, emerged as a severe acute atypical respiratory infection that ravaged the Wuhan city of Hubei province of China in December 2019 (Wang et al. 2021). The pathogen responsible for these atypical infections was soon discovered as a novel coronavirus belonging to the family Coronaviridae and was named the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). It was found to be quite similar to the SARS coronavirus (SARS-CoV), which caused the respiratory pandemic in 2002–2003. The outbreak of SARS-CoV-2 was originally started via a zoonotic transmission associated with the seafood market in Wuhan, China. Later, the human-to-human transmission was subsequently found to have had a crucial role in the ensuing outbreak. Coronavirus disease 2019 (COVID-19) was caused by this virus, and the World Health Organization (WHO) proclaimed it a pandemic. Also, it has impacted a vast number of people globally, with reports from approximately 200 nations and territories (Yang et al. 2019).
The SARS-CoV-2 virus primarily affects the respiratory system, although other organ systems are also involved. In the first case series from Wuhan, China, symptoms associated with lower respiratory tract infections such as fever, dyspnea, and dry cough were observed. In addition to that, dizziness, headache, diarrhea, vomiting, and generalized weakness were reported. COVID-19 respiratory symptoms are now well acknowledged to be quite diverse, ranging from mild symptoms to severe hypoxia with acute respiratory distress syndrome (ARDS) (Amin MT et al. 2021). In the report from Wuhan mentioned above, the time between the onset of symptoms and acute respiratory distress syndrome (ARDS) development was as short as 9 days, suggesting that the respiratory symptoms could progress rapidly (Li et al. 2020). Patients with severe conditions inevitably die in increasing numbers around the world. As the outbreak continued, the number of cases among people aged 65 years and older increased further, but some increase among children (< 18 years) was observed. The number of male patients was higher at first, but there were no significant differences as the number of cases grew. The average time for incubation was 5.2 days. (Wu Y et al. 2022).
Coronaviruses consist of four structural proteins; spike (S), membrane (M), envelop (E), and nucleocapsid (N). Spike is a transmembrane trimetric glycoprotein that protrudes from the viral surface and controls coronavirus diversification and host tropism. Spike is made up of two functional subunits; the S1 subunit is essential for attaching to the host cell receptor, while the S2 subunit is important for fusing the cellular and viral membranes. Angiotensin-converting enzyme 2 (ACE2) was identified as a functional receptor for SARS-CoV (J. Zhang et al. 2020a, b). Structural and functional analysis showed that the spike for SARS-CoV-2 was also bound to ACE2. ACE2 expression was high in the lung, heart, ileum, kidney, and bladder (Li et al. 2003). On lung epithelial cells, ACE2 was shown to be significantly expressed. After SARS-CoV-2 binds to ACE-2, the S protein is activated by a two-step protease cleavage: the first one for priming at the S1/S2 cleavage site and the second cleavage for activation at a position adjacent to a fusion peptide within the S2 subunit. The first cleavage stabilizes the S2 subunit at the attachment point, whereas the second cleavage activates the S protein, triggering conformational changes that lead to viral and host cell membrane fusion. The furin cleavage site (“RPPA” sequence) at the S1/S2 site distinguishes SARS-CoV-2 from other coronaviruses. The S1/S2 site of SARS-CoV-2 was entirely subjected to cleavage during biosynthesis in drastic contrast to the SARS-CoV spike, which was incorporated into the assembly without cleavage (Walls et al. 2020). Although the S1/S2 site was also subjected to cleavage by other proteases such as transmembrane protease serine 2 (TMPRSS2) and cathepsin L, the ubiquitous expression of furin likely makes this virus very pathogenic. SARS-CoV-2 attaches to nasal epithelial cells in the upper respiratory tract after being inhaled by respiratory aerosols. The ACE-2 receptor is the primary host receptor for viral entrance into cells, and it is found to be abundantly expressed in adult nasal epithelial cells. The virus replicates and spreads locally in the conducting airways, infecting ciliated cells (Ou et al. 2020). This stage lasts a couple of days, and the immune response generated during this phase is limited. Despite having a low viral load at this time, the individuals are highly infectious, and the virus can be detected via nasal swab testing. Because ACE2 is strongly expressed on the apical side of lung epithelial cells in the alveolar space (Huang et al. 2020), this virus has a good chance of infecting and destroying them. This corresponds to the fact that initial lung impairment was frequently observed in the distal airway.
The three main components of innate immunity in the airway are epithelial cells, alveolar macrophages, and dendritic cells (DCs). DCs reside underneath the epithelium. Macrophages are found on the epithelium’s apical side. Until adaptive immunity is involved, DCs and macrophages act as innate immune cells, fighting viruses. Antigen presentation through DCs and macrophages triggers T cell responses. DCs and macrophages can phagocytize apoptotic cells infected by the virus. For example, DCs and macrophages can phagocytize virus-infected apoptotic epithelial cells, resulting in antigen presentation to T cells. SARS-CoV can also bind to dendritic-cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) and DC-SIGN-related protein (DC-SIGNR, L-SIGN) in addition to ACE2 (Sims et al. 2005). On dendritic cells and macrophages, DC-SIGN is highly expressed. This needs future research. These antigen-presenting cells move to the draining lymph nodes to present viral antigens to T cells. CD4 + and CD8 + T cells play a critical role. CD4 + T cells activate B cells to promote the production of virus-specific antibodies, while CD8 + T cells can kill virally infected cells.
Immunological investigations were mostly reported in COVID-19 individuals who were infected with the virus. Patients with severe diseases showed lymphopenia, particularly the reduction in peripheral blood T cells. Patients with severe diseases were reported to have increased plasma concentrations of proinflammatory cytokines, including interleukin (IL)-6, IL-10, granulocyte-colony stimulating factor (G-CSF), monocyte chemoattractant protein 1 (MCP1), macrophage inflammatory protein (MIP) 1α, and tumor necrosis factor (TNF)-α (Yoshikawa et al. 2009). The higher the IL-6 levels, the more serious the patient’s condition. CD4 + and CD8 + T cells were activated in those patients as suggested by higher expression of CD69, CD38, and CD44. The higher percentage of checkpoint receptor Tm3 + PD-1 + subsets in CD4 + and CD8 + T cells showed that T cells were also exhausted. NK group 2 member A (NKG2A), another marker for exhaustion, was elevated on CD8 + T cells. T cell exhaustion may have accelerated the disease’s progression. Another interesting finding was that aberrant pathogenic CD4 + T cells with co-expressing interferon (IFN)-γ and granulocyte–macrophage colony-stimulating factor (GM-CSF) were seen in COVID-19 patients with severe disease. T cells have previously been shown to produce GM-CSF in response to viral infection. GM-CSF can help to differentiate innate immune cells and augment T cell function, but it can initiate tissue damage at excess (Fujimoto et al. 2000). GM-CSF + IFN-γ + CD4 + T cells were previously seen upon strong T cell receptor (TCR) responses in experimental autoimmune encephalomyelitis (EAE) models, where CD8 + T cells expressing GM-CSF were found at a higher percentage and secreted IL-6 (Heinrich et al. 1990). The study of SARS-CoV showed that virus-infected lung epithelial cells produced IL-8 in addition to IL-6 (Fig. 1).
For neutrophils and T lymphocytes, IL-8 is a well-known chemoattractant. Infiltration of a large number of inflammatory cells was observed in the lungs from severe COVID-19 patients, and these cells presumably consist of a constellation of innate immune cells and adaptive immune cells. Neutrophils should account for the majority of innate immune cells. Neutrophils can act as a double-edged sword as neutrophils can induce lung injury. Most of the observed infiltrating adaptive immune cells were likely T cells, considering that the significant reduction in circulating T cells was reported. CD8 + T cells are primary cytotoxic T cells. Severe patients also showed pathological cytotoxic T cells derived from CD4 + T cells. These cytotoxic T cells can kill the virus but also contribute to lung injury (Croxford et al. 2015). The pathogenic T cells secrete GM-CSF, which is recognized by circulating monocytes. CD14 + CD16 + inflammatory monocyte subsets, which seldom exist in healthy controls and were also found at a significantly higher percentage in COVID-19 patients.
These inflammatory CD14 + CD16 + monocytes had high expression of IL-6, which likely accelerated the progression of systemic inflammatory response. Interestingly, ACE2 was significantly expressed on innate lymphoid cells (ILC)2 and ILC3. ILC1 includes NK cells, constituting a major portion of ILCs in the lungs (95 percent). ILC2 and ILC3 work for mucous homeostasis.
Role of kinases in SARS-CoV-2
Cytokines and interleukins are defined as collective polypeptide signaling molecules playing a major role in immunity and inflammation processes. In microbial infections and cancers, cytokines are being synthesized by the host cells as a defensive response. Among them IL-2 and IL-4 are involved in adaptive immunity; (IFN)-I, (IFN)-II, and (IFN)-III; IL-1, IL-6, and IL-17; and TNF-α in proinflammatory regulation; and anti-inflammatory cytokines (e.g., IL-10). The abnormal levels of the following cytokines and chemokines in the COVID-19 patients were detected: IL-1, IL-2, IL-4, IL-6, IL-7, IL-10, IL-12, IL-13, IL-17, M-CSF, G-CSF, GM-CSF, IP-10, IFN-γ, MCP-1, MIP 1-α, hepatocyte growth factor (HGF), TNF-α, and vascular endothelial growth factor (VEGF) (Wong et al. 2004). This elevation of cytokines leading to the depletion of host cell defense mechanism could be considered as the key pathological process in COVID-19. The cellular kinases have prominence in each stage of viral replication in SARS-CoV-1 and SARS-CoV-2 and MERS-CoV. In CoV-1, the endosomal membrane fusion is mediated by Abelson (Abl) kinases and replication is mediated by the actions of p38 MAPK and JNK1/2 kinases. Symptoms like inflammation of lung alveoli, pneumonia, and fibrosis are related to the synthesis and regulation of IL6, IL10, and TNF alpha like harmful kinases by the Abl and MAPK pathways. This is the main reason for the employment of kinase inhibitors in COVID.
IL-1 and its antagonists in COVID-19
The IL-1 activates monocytes and macrophages and thus has an extensive participation in the inflammatory response to infection. Initial activation and maturation of IL-1β by SARS-CoV-2 lead to cytokine storm by activating IL-6 and TNF-α proinflammatory cytokines. Elevation of IL-1Ra (the IL-1 antagonistic receptor) in the serum was identified in 14 severe cases of COVID-19 patients and was responsible for increase in viral load, loss of pulmonary function, lung damage, and mortality risk (Costela-Ruiz et al. 2020). Elevation in IL-1β is also responsible for hyper coagulation and disseminated intravascular coagulation. In this way, mesenchymal stem cells (MSCs) have been used to inhibit proinflammatory cytokines such as IL-1α and TNF-α (Small et al. 2001) (Fig. 2).
Anakinra (Brand name—Kineret), IL-1 inhibitor, has gained interest in COVID-19 treatment initially, but its efficacy was not studied in controlled trials. Moreover, Anakinra did not show any improvement in the mild-to-moderate conditions of COVID. IL-1 antibody antagonists, namely canakinumab and rilonacept, are being studied in the treatment in cytokine storm infection.
IL-6 Inhibitors mediated through Abl and Src kinases
IL-6 is a cytokine that plays major part in the inflammation pathways, immune responses, and hematopoiesis. Its actions are responsible for the production and release of C-reactive protein (CRP), serum amyloid A (SAA), fibrinogen, haptoglobin, and α1-antitrypsin. The production of fibronectin, albumin, and transferrin proteins was depleted by IL-6. Elevated IL-6 levels and T cell dysfunctioning have been observed in different SARS infections and related to the severity of symptoms like cytokine storm and inflammatory process activation. IL-6 and CRP were seen elevated in 80 percent of COVID-19 patients with severe symptoms and in 15–20 percent mild symptomatic patients. High IL-6 lead to decreased mortality in COVID-19 patients as it as studies show IL-6 was also found to be markedly higher in patients who died from COVID-19 than in those who recovered. The cytokine storm, including elevated levels of IL-6, has also been associated with cardiac damage in these patients (Tang et al. 2020) (Fig. 3).
The activation of IL6 is through Abl kinases which play role in stress responses. Hence, this pathway has proved to be an important pathological reason for coronavirus replication. Three kinase signaling pathway inhibitors, namely imatinib, dasatinib, and nilotinib, were found to be effective in the treatment against SARS-CoV-1 and MERS-CoV. Imatinib and dasatinib are widely employed as an orally available Abl kinase inhibitor in chronic myeloid leukemia (CML) and acute lymphoblastic leukemia. Imatinib was discovered to block the entry and replication of SARS-CoV-1 virus by preventing the fusion of virions at endosomal membranes and hence could be effective in early COVID condition. This mechanism of imatinib was effective in inhibiting Ebola virus, poxvirus, and coxsackievirus (Dyall et al. 2014). Imatinib has immunomodulatory effects and could suppress the cytokine storms by arresting the cytokine receptor signaling (PDGFR, c-Kit, and CSF1R0). Since SARS-CoV-1 and SARS-CoV-2 have a high degree of genome sequence identity (80%), imatinib is predicted to inhibit SARS-CoV-2 as well. Nilotinib is a structural analog of imatinib inhibits the SARS-CoV-1 replication in the micromolar range by blockade of the Abl kinase pathway, and their actions are mediated through Abl2 pathway. The Abl2 kinase has 2 domains, namely SH2 and SH3; binding of the substrate to either of the domain will activate the ATP generation, resulting in the phosphorylated activation of Abl2 kinase activity in COVID. Imatinib successfully inhibits Abl2 activity and thus prevents the phosphorylation of proteins (Fig. 4).
The process of viral replication begins with receptor binding, S glycoprotein activation, proteolysis, and the entry of virus into the host cells. Cathepsin L mediates the proteolysis, and TMPRSS2 helps in the activation of S glycoprotein resulting in the entry of coronavirus into host cells. Cathepsin inhibitors [EST-(23,25)trans-epoxysuccinyl-l-leucylamindo-3-methylbutane ethyl ester] and serine glycoprotein inhibitor (Camostat) are also proposed in coronavirus disease to prevent the viral entry and replication. Imatinib has the action of inhibiting cathepsin and S glycoproteins.
Numb-associated kinases and inhibitors
This group of viruses has 2 human analogues, AAK1 (AK1 adaptor protein-associated kinase) and GAK (cyclin G-associated kinase), which has shown their prominence in replication of several dreadful viruses like dengue, Ebola, SARS-COV-1, SARS-COV-2, and MERS-COV. Sunitinib and erlotinib suppress the AAK1 and GAK by inhibiting the replication of virus. JAK inhibitors have established their effectiveness in the management of rheumatoid arthritis, psoriatic arthritis, ulcerative colitis, and myeloproliferative malignancies. Baricitinib at 4 mg/day among the JAK inhibitors has proved its efficacy in arresting the alveolar (AT2) viral replication by inhibiting the AAK1 (AK1 adaptor protein-associated kinase) and GAK (cyclin G-associated kinase) pathways and also shows reduction in the inflammation of alveoli cells. ACE2 is mediated by this AAK1 and GAK pathways. Ruxolitinib and fedratinib reduce the viral infectivity at loaded doses, and tofacitinib has no significance in AAK1 pathway. A randomized clinical study performed by Kalmanti et al. (Kalmanti et al. 2015) with 1033 hospitalized patients affected by COVID-19 exhibits the efficacy of JAK inhibitors in combination therapy with anti-virals. 555 SARS pneumonia patients were treated baricitinib 4 mg/day for two weeks and remdesivir and 518 receiving remdesivir alone. Shorter recovery time with less mortality was observed in the patients’ receiving combination therapy.
Inhibitors of cyclin-dependent kinases (CDKs)
CDKs are mainly targeted for anticancer agents and certain anti-viral treatments. HIV, Hepatitis, herpes, and Zika viruses were related to CDKs pathway. CDK1 and 2 actions were inhibited by SARS-COV-2, thereby arresting the S/G2 cell cycle phase, and subsequently boost the viral life cycle. Dinaciclib has proven to show anti-SARS-COV-2 action in cell lines (Vero E6, A549-ACE2). Abemaciclib is a selective CDK4/6 inhibitor which was studied to inhibit the coronavirus cytopathic effect (CPE) by blocking the entry of virus into the host cell.
Inhibitors of AXL kinases in COVID-19
This tyrosine kinase mediates the cell replication, growth and differentiation process and immunity process. It stimulates various signaling pathways like JAK/STAT, p38, MEK-ERK, RAS/ERK. AXL serves as an immune modulator in Ebola and Zika viral replications. In SARS-COV-2, it interacts with the S glycoprotein on host cell and mediates the entry of virus in to the host cells (pulmonary and bronchial epithelial cells). AXL inhibitor gilteritinib was studied to inhibit the entry of SARS-COV-2 virus in to the pulmonary host cells.
Src kinases in COVID-19
These are very crucial in the signal transduction and stimulate cell synthesis and motility. These take part in the replication of dengue virus, hepatitis C, coronaviruses. Dasatinib and saracatinib inhibit Src kinases pathway in MERS-CoV in early stage of replication (Thobe et al. 2006).
Anti-IL-6 receptor monoclonal antibodies (mAbs), namely sarilumab, tocilizumab, and siltuximab, were approved by the Food and Drug Administration. Tocilizumab effectively reverse the cytokine storm, pulmonary fibrosis, and alveoli inflammation by inhibition of IL-6 receptors. Tocilizumab and sarilumab should only be given in combination with a course of dexamethasone or an alternative corticosteroid. The polyol myo-inositol also inhibits IL-6 levels and the risk of cytokine storm. Azithromycin in combination with hydroxychloroquine also has the capacity to block IL-6 and TNF-α. Chloroquine also has the capacity to inhibit IL-6 and TNF-α and is being tested against COVID-19 (Dholaria et al. 2019).
P38 signaling pathway and its inhibitors in COVID-19
Environmental stress, pathogenic infections, and proinflammatory cytokines will activate the p38 signaling pathway in cancers and viral infections, thus leading to cell apoptosis and autophagy. In SARS-COV-1, activation of p38 pathway is resulted in pathogenic inflammatory responses. Inflammation-mediated ARDS and myocarditis are related to deaths in serious COVID patients (Gautret et al. 2020). Activation of p38 pathway results in the synthesis and responses of IL6, TNFs leading to inflammatory injury in SARS-COV-2 patients. SARS-COV-1 and SARS-COV-2 invade the host cells by using ACE2 and cause disturbance in the renin–angiotensin–aldosterone system in host cells. ACE2 has an important role in conversion of angiotensin II to angiotensin I, which leads to induction of anti-inflammatory, anti-fibrinogenic, and vasodilator actions (mediated by p38 downregulation). Activation of p38 by angiotensin II is responsible for proinflammation and prothrombotic actions.
Hence, decrease in the ACE2 actions in COVID infections leads to p38 MAPK activation-mediated inflammatory responses by producing Ang 1–7 in heart and lungs. A p38 MAPK inhibitor SB2033580 shows reduction in the levels IL6 and TNF in COVID patients. It also reduced the replication of SARS-COV-2 by arresting the viral cell SA/G2 phases. Gilteritinib (AXL inhibitor), ralimetinib (MAPK inhibitor), and ginsenoside inhibit the viral replication significantly by suppressing p38/JAK/MAPK signaling pathway. P38 MAPK signaling was shown to viral replication by inducing the ACE2 receptor-mediated endocytosis. This gives an evidence that SARS-COV is related to the activation of p38 MAPK pathway leading to inflammatory responses in respiratory viral infections and also provides a justification to the application of p38 inhibitors in COVID-19 treatment.
Role of JAK-STAT signaling pathway in COVID-19 and its inhibitors
The IFNs get bound to their specific receptors and cause activation of Janus kinases, and activators of JAK-STAT signaling pathways mediate specific cell signaling mechanisms involving cytokines and growth factors. JAK-STAT pathway takes active role in cell growth and differentiation, biological processes, and oxidative stress. In certain viruses, HCV protein interacts with JAK1 during replication process (for example, in HIV virus, JAK-STAT pathway stimulates IL-2 by T cell activation) (Fang et al. 2017) (Fig. 5).
In coronaviruses, the factors, namely SARS-COV-1 NSP1 and ORF6, mediate the IFN production and response. This results in the inhibition of JAK-STAT1 pathway by promoting the activity of STAT3. STAT3 causes stimulation of IL6 leading to pulmonary fibrosis in COVID patients. Multiple organ failure and lung fibrosis in COVID-19 patients are due to the activation and release of IL-1, IL-2, IL-6, IL-7, IL-10, TNF, etc. This evidence has suggested that the serious conditions in COVID patients (organ failure, cytokine storm, fibrosis, etc.) could be prevented by inhibiting JAK-STAT pathway which in turn blocks the release and actions of these cytokines (Kang et al. 2018). JAK-STAT inhibitors, namely ruxolitinib, baricitinib, tofacitinib, oclacitinib, upadacitinib, and fedratinib, were being employed as successful anti-inflammatory agents in rheumatoid arthritis, psoriasis, and other auto-immune disorders.
Inhibition of JAK-STAT pathway is the first step in preventing severe stages in SARS-COV-2 infection. But delay in the IFN-1 treatment leads to suppression of viral infection and induces cytokine synthesis resulting in the pneumonia. IFN-1 nasal drops with thymosin 1 prevented the respiratory infection. Tofacitinib, an oral anti-JAK drug, acts by inhibiting JAK3 and TYK2 and shows anti-inflammatory action in several auto-immune disorders and also showed its efficiency in COVID patients with ulcerative colitis. Another study shows fedratinib, a JAK2 inhibitor, is effective in management of myelofibrosis in COVID-19 by suppressing TH17-associated cytokine activation. TH17 produces IL-17, and hence, fedratinib inhibits the release and actions of IL-17 thereby preventing cytokine storm. Ruxolitinib also inhibits several cytokines release by arresting the STAT3 pathway associated with myelofibrosis in COVID, thereby inhibiting the serious cytokine storm (Xiong et al. 2020). In 20 clinical trials associated with ruxolitinib, the clinical development was good compared to the control group patients. Hematological toxicity and herpes virus reactivation are the serious side effects with JAK-STAT inhibitors and lead to the suspension of ruxolitinib from COVID-19 treatment.
Glycogen synthase kinase-3 signaling pathway
Glycogen synthase kinase-3 exists in alpha and beta forms and plays an important role in inflammatory reactions, cellular metabolism, and development of innate immunity. Hence, drugs targeting this pathway has gained importance in the management of several cancers, neurodegenerative disorders, bipolar disorders, and diabetes. GSK-3 takes part in mediation of gene transcription by leading to the activation of ILs, STAT3, CREB, and NFAT, NF-B. The importance of this GSK-3 pathway is less studied in viral replication process. This pathway has a prominent place in replication of certain viruses and host immunity. It has been identified as a possible mediator in the synthesis, formation, and functioning of HCV virions (Ingraham et al. 2020). Studies have shown that inhibition of GSK-3 reduced the viral replication in HIV-1 cell lines.
The nucleocapsid protein N, a structural protein of coronaviruses, takes part in the formation of a protective coating to the viral genomic RNA during replication process. Thus, the virus will be strongly incorporated into the host cells and host immunity will be affected. SARS-COV-1 N-phosphorylation, a major step in viral replication, is also mediated by GSK-3. GSK-3 pathway has importance in phosphorylation step of both SARS-COV-1 and SARS-COV-2, as both the virus consists of similar N protein. Hence, drugs that could inhibit the GSK-3 would also prevent the N-phosphorylation step in the replication of coronaviruses (Mullard 2018). Moreover, inhibiting the GSK-3 signaling pathway helps in the synthesis of natural killer cells resulting in the development of innate immunity. Lithium chloride and kenpaullone were studied to reduce the viral growth and cytopathic effects in the Vero E6 cells incubated with SARS-COV-1. Recent study shows the anti-SARS-COV-2 action of Tideglusib.
Tumor necrosis factor α in COVID-19
TNF-α is a cytokine synthesized in monocytes, macrophages, and T cells and associated with the proinflammatory responses mediated by IL-1β and IL-6 by regulating the inflammatory processes, infectious diseases, and malignant tumors. The serum TNF-α levels are elevated in COVID-19 patients. Over-production of TNF-α leads to a poor prognosis in patients with SARS-CoV and MERS. An anti-TNF-α antibody, certolizumab, was administered to COVID-19 patients and has shown beneficial effects (Mahallawi et al. 2018; R. Zhang et al. 2020a, b) (Fig. 6).
Conclusion
Kinase plays a vital role in the immune response, inflammation, and host defense mechanism. Numerous kinases is actively involved in the signaling cascade of COVID as well as cancer. Hence, future directions can be focused on discovering kinase inhibitors which can be helpful in the treatment of inflammatory diseases as well as cancers. Over the past 35 years, there have been significant improvements in the understanding of RTK signaling pathways. Basic research on the genetics, cellular biology, biochemistry, and structural biology of these receptors has developed quite an advanced understanding about the functioning of this huge family of proteins. These discoveries are outstanding instances of laboratory-driven translational research and have contributed to the development of several significant therapeutics. Importantly, clinical studies of RTKs in disease have offered significant mechanistic understanding and provide opportunity for the further development of therapeutic approaches. The chemical pathways underlying RTK activation are similar across families but vary significantly in their specific functions. The completion of the RTK families, of which only about half are fully characterized, is a significant challenge for the future, and this will undoubtedly show us many new discoveries about RTK activation and signaling. The significance of direct receptor cross talk or heterodimerization for signaling specificity at the level of the receptor molecules themselves is still unclear, as is the precise function of intracellular trafficking. Another significant difficulty for the future is gaining quantitative knowledge of these problems. Finally, it becomes increasingly obvious that a quantitative biochemical understanding of the behavior of the systems is essential for forecasting qualitative outcomes as we begin to appreciate the geographical, temporal, and chemical complexity of the signaling networks regulated by RTKs. Although many signaling components in RTK networks have been characterized by genetic and pharmacological methods, placing these components in mechanistic and quantitative contexts represents the next significant hurdle. Hence, new, efficient treatments for COVID-19 and other conditions caused by active RTKs can therefore be developed.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Abourehab MAS, Alqahtani AM, Youssif BGM, Gouda AM (2021) Globally approved EGFR inhibitors: insights into their syntheses, target kinases, biological activities, receptor interactions, and metabolism. Molecules 26(21):6677. https://doi.org/10.3390/molecules26216677.PMID:34771085;PMCID:PMC8587155
Aksamitiene E, Kiyatkin A, Kholodenko BN (2012) Cross-talk between mitogenic Ras/MAPK and survival PI3K/Akt pathways: a fine balance. Biochem Soc Trans 40:139–146. https://doi.org/10.1042/BST20110609
Alexander PB, Wang X-F (2015) Resistance to receptor tyrosine kinase inhibition in cancer: molecular mechanisms and therapeutic strategies. Front Med 9:134–138. https://doi.org/10.1007/s11684-015-0396-9
Amin MT, Hasan M, Bhuiya NMMA (2021) Prevalence of Covid-19 associated symptoms, their onset and duration, and variations among different groups of patients in Bangladesh. Front Public Health 9:738352. https://doi.org/10.3389/fpubh.2021.738352
Anafi M, Gazit A, Zehavi A et al (1993) Tyrphostin-induced inhibition of p210bcr-abl tyrosine kinase activity induces K562 to differentiate. Blood 82:3524–3529. https://doi.org/10.1182/blood.V82.12.3524.bloodjournal82123524
Bhullar KS, Lagarón NO, McGowan EM et al (2018) Kinase-targeted cancer therapies: progress, challenges and future directions. Mol Cancer 17:48. https://doi.org/10.1186/s12943-018-0804-2
Blanke CD, Corless CL (2005) State-of-the art therapy for gastrointestinal stromal tumors. Cancer Invest 23:274–280. https://doi.org/10.1081/CNV-200055972
Blanke CD, Demetri GD, von Mehren M et al (2008) Long-term results from a randomized phase II trial of standard-versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol 26:620–625. https://doi.org/10.1200/JCO.2007.13.4403
Brose MS, Nutting CM, Jarzab B et al (2014) Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 384:319–328. https://doi.org/10.1016/S0140-6736(14)60421-9
Cardoso F, Senkus E, Costa A et al (2018) 4th ESO–ESMO international consensus guidelines for advanced breast cancer (ABC 4). Ann Oncol 29:1634–1657. https://doi.org/10.1093/annonc/mdy192
Carlo-Stella C, Regazzi E, Sammarelli G et al (1999) Effects of the tyrosine kinase inhibitor AG957 and an anti-Fas receptor antibody on CD34+ chronic myelogenous leukemia progenitor cells. Blood 93:3973–3982. https://doi.org/10.1182/blood.V93.11.3973
Casali PG, Abecassis N, Bauer S et al (2018) Gastrointestinal stromal tumours: ESMO–EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 29:iv68–iv78. https://doi.org/10.1093/annonc/mdy095
Chakraborty HJ, Gangopadhyay A, Datta A (2019) Prediction and characterisation of lantibiotic structures with molecular modelling and molecular dynamics simulations. Sci Rep 9:1–11. https://doi.org/10.1038/s41598-019-42963-8
Chan X, Singh A, Osman N, Piva T (2017) Role played by signalling pathways in overcoming BRAF inhibitor resistance in melanoma. Int J Mol Sci 18:1527. https://doi.org/10.3390/ijms18071527
Chandra J (2006) Adaphostin-induced oxidative stress overcomes BCR/ABL mutation-dependent and-independent imatinib resistance. Blood 107:2501–2506. https://doi.org/10.1182/blood-2005-07-2966
Checkley D, Tessier JJ, Kendrew J et al (2003) Use of dynamic contrast-enhanced MRI to evaluate acute treatment with ZD6474, a VEGF signalling inhibitor, in PC-3 prostate tumours. Br J Cancer 89:1889–1895. https://doi.org/10.1038/sj.bjc.6601386
Cooke M, Magimaidas A, Casado-Medrano V, Kazanietz MG (2017) Protein kinase C in cancer: the top five unanswered questions. Mol Carcinog 56:1531–1542. https://doi.org/10.1002/mc.22617
Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L (2020) SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev 54:62–75. https://doi.org/10.1016/j.cytogfr.2020.06.001
Crisci S, Amitrano F, Saggese M et al (2019) Overview of current targeted anti-cancer drugs for therapy in onco-hematology. Medicina (b Aires) 55:414. https://doi.org/10.3390/medicina55080414
Croxford AL, Lanzinger M, Hartmann FJ et al (2015) The cytokine GM-CSF drives the inflammatory signature of CCR2+ monocytes and licenses autoimmunity. Immunity 43:502–514. https://doi.org/10.1016/j.immuni.2015.08.010
Denny WA (2001) The 4-anilinoquinazoline class of inhibitors of the erbB family of receptor tyrosine kinases. Farm 56:51–56. https://doi.org/10.1016/S0014-827X(01)01026-6
Dholaria BR, Bachmeier CA, Locke F (2019) Mechanisms and management of chimeric antigen receptor T-Cell therapy-related toxicities. BioDrugs 33:45–60. https://doi.org/10.1007/s40259-018-0324-z
Ding Y, Gong C, Huang D et al (2018) Synthetic lethality between HER2 and transaldolase in intrinsically resistant HER2-positive breast cancers. Nat Commun 9:4274. https://doi.org/10.1038/s41467-018-06651-x
Discafani CM, Carroll ML, Floyd MB et al (1999) Irreversible inhibition of epidermal growth factor receptor tyrosine kinase with In Vivo activity by N-[4-[(3-bromophenyl)amino]-6-quinazolinyl]-2-butynamide (CL-387,785). Biochem Pharmacol 57:917–925. https://doi.org/10.1016/S0006-2952(98)00356-6
Dvorak HF (2003) How tumors make bad blood vessels and stroma. Am J Pathol 162:1747–1757. https://doi.org/10.1016/S0002-9440(10)64309-X
Dyall J, Coleman CM, Hart BJ et al (2014) Repurposing of clinically developed drugs for treatment of middle East respiratory syndrome coronavirus infection. Antimicrob Agents Chemother 58:4885–4893. https://doi.org/10.1128/AAC.03036-14
Escudier B, Porta C, Schmidinger M et al (2019) Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 30:706–720. https://doi.org/10.1093/annonc/mdz056
Fabbro D, Cowan-Jacob SW, Moebitz H (2015) Ten things you should know about protein kinases: IUPHAR Review 14. Br J Pharmacol 172:2675–2700. https://doi.org/10.1111/bph.13096
Fang W, Cai S-X, Wang C-L et al (2017) Modulation of mitogen-activated protein kinase attenuates sepsis-induced acute lung injury in acute respiratory distress syndrome rats. Mol Med Rep 16:9652–9658. https://doi.org/10.3892/mmr.2017.7811
Fong TA, Shawver LK, Sun L et al (1999) SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res 59:99–106
Fouad YA, Aanei C (2017) Revisiting the hallmarks of cancer. Am J Cancer Res 7:1016–1036
Fujimoto I, Pan J, Takizawa T, Nakanishi Y (2000) Virus clearance through apoptosis-dependent phagocytosis of influenza a virus-infected cells by macrophages. J Virol 74:3399–3403. https://doi.org/10.1128/JVI.74.7.3399-3403.2000
Gargouri M, Alzwi A, Abobaker A (2021) Cyclin dependent kinase inhibitors as a new potential therapeutic option in management of COVID-19. Med Hypotheses 146:110380. https://doi.org/10.1016/j.mehy.2020.110380
Gautret P, Lagier J-C, Parola P et al (2020) Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 56:105949. https://doi.org/10.1016/j.ijantimicag.2020.105949
Gazit A, Yaish P, Gilon C, Levitzki A (1989) Tyrphostins I: synthesis and biological activity of protein tyrosine kinase inhibitors. J Med Chem 32:2344–2352. https://doi.org/10.1021/jm00130a020
Greene CS, Krishnan A, Wong AK, Ricciotti E, Zelaya RA, Himmelstein DS, Zhang R, Hartmann BM, Zaslavsky E, Sealfon SC, Chasman DI, FitzGerald GA, Dolinski K, Grosser T, Troyanskaya OG (2015) Understanding multicellular function and disease with human tissue-specific networks. Nat Genet 47(6):569–576. https://doi.org/10.1038/ng.3259
Grothey A, Van CE, Sobrero A et al (2013) Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381:303–312. https://doi.org/10.1016/S0140-6736(12)61900-X
Heinrich PC, Castell JV, Andus T (1990) Interleukin-6 and the acute phase response. Biochem J 265:621–636. https://doi.org/10.1042/bj2650621
Hennequin LF, Stokes ESE, Thomas AP et al (2002) Novel 4-Anilinoquinazolines with C-7 basic side chains: design and structure activity relationship of a series of potent, orally active, VEGF receptor tyrosine kinase inhibitors. J Med Chem 45:1300–1312. https://doi.org/10.1021/jm011022e
Hochmair MJ, Morabito A, Hao D et al (2019) Sequential afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: updated analysis of the observational GioTag study. Futur Oncol 15:2905–2914. https://doi.org/10.2217/fon-2019-0346
Huang C, Wang Y, Li X et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497–506. https://doi.org/10.1016/S0140-6736(20)30183-5
Ingraham NE, Lotfi-Emran S, Thielen BK et al (2020) Immunomodulation in COVID-19. Lancet Respir Med 8:544–546. https://doi.org/10.1016/S2213-2600(20)30226-5
Kalmanti L, Saussele S, Lauseker M et al (2015) Safety and efficacy of imatinib in CML over a period of 10 years: data from the randomized CML-study IV. Leukemia 29:1123–1132. https://doi.org/10.1038/leu.2015.36
Kang S, Song MJ, Min H (2018) Antiviral activity of ginsenoside Rg3 isomers against gammaherpesvirus through inhibition of p38- and JNK-associated pathways. J Funct Foods 40:219–228. https://doi.org/10.1016/j.jff.2017.11.011
Katoh M (2016) FGFR inhibitors: effects on cancer cells, tumor microenvironment and whole-body homeostasis (review). Int J Mol Med 38:3–15. https://doi.org/10.3892/ijmm.2016.2620
Kimura H, Fujiwara Y, Sone T et al (2006) EGFR mutation status in tumour-derived DNA from pleural effusion fluid is a practical basis for predicting the response to gefitinib. Br J Cancer 95:1390–1395. https://doi.org/10.1038/sj.bjc.6603428
Kovalenko M, Gazit A, Böhmer A et al (1994) Selective platelet-derived growth factor receptor kinase blockers reverse sis-transformation. Cancer Res 54:6106–6114
Kralovics R, Passamonti F, Buser AS et al (2005) A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med 352:1779–1790. https://doi.org/10.1056/NEJMoa051113
Lacal PM, Graziani G (2018) Therapeutic implication of vascular endothelial growth factor receptor-1 (VEGFR-1) targeting in cancer cells and tumor microenvironment by competitive and non-competitive inhibitors. Pharmacol Res 136:97–107. https://doi.org/10.1016/j.phrs.2018.08.023
Laird AD, Vajkoczy P, Shawver LK et al (2000) SU6668 is a potent antiangiogenic and antitumor agent that induces regression of established tumors. Cancer Res 60:4152–4160
Lei HY, Ding YH, Nie K, Dong YM, Xu JH, Yang ML, Liu MQ, Wei L, Nasser MI, Xu LY, Zhu P, Zhao MY (2021) Potential effects of SARS-CoV-2 on the gastrointestinal tract and liver. Biomed Pharmacother 133:111064. https://doi.org/10.1016/j.biopha.2020.111064
Lemmon MA, Schlessinger J (2010) Cell signaling by receptor tyrosine kinases. Cell 141:1117–1134. https://doi.org/10.1016/j.cell.2010.06.011
Li W, Moore MJ, Vasilieva N et al (2003) Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426:450–454. https://doi.org/10.1038/nature02145
Li Q, Guan X, Wu P et al (2020) Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 382:1199–1207. https://doi.org/10.1056/NEJMoa2001316
Liang F, Ren C, Wang J et al (2019) The crosstalk between STAT3 and p53/RAS signaling controls cancer cell metastasis and cisplatin resistance via the Slug/MAPK/PI3K/AKT-mediated regulation of EMT and autophagy. Oncogenesis 8:59. https://doi.org/10.1038/s41389-019-0165-8
Liebmann C (2001) Regulation of MAP kinase activity by peptide receptor signalling pathway: paradigms of multiplicity. Cell Signal 13:777–785. https://doi.org/10.1016/S0898-6568(01)00192-9
Mahallawi WH, Khabour OF, Zhang Q et al (2018) MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine 104:8–13. https://doi.org/10.1016/j.cyto.2018.01.025
Maifrede S, Nieborowska-Skorska M, Sullivan-Reed K et al (2018) Tyrosine kinase inhibitor–induced defects in DNA repair sensitize FLT3(ITD)-positive leukemia cells to PARP1 inhibitors. Blood 132:67–77. https://doi.org/10.1182/blood-2018-02-834895
McCarty MF, Wey J, Stoeltzing O et al (2004) ZD6474, a vascular endothelial growth factor receptor tyrosine kinase inhibitor with additional activity against epidermal growth factor receptor tyrosine kinase, inhibits orthotopic growth and angiogenesis of gastric cancer. Mol Cancer Ther 3:1041–1048. https://doi.org/10.1158/1535-7163.1041.3.9
McTigue MA, Wickersham JA, Pinko C et al (1999) Crystal structure of the kinase domain of human vascular endothelial growth factor receptor 2: a key enzyme in angiogenesis. Structure 7:319–330. https://doi.org/10.1016/S0969-2126(99)80042-2
Mishani E, Abourbeh G, Jacobson O et al (2005) High-affinity epidermal growth factor receptor (EGFR) irreversible inhibitors with diminished chemical reactivities as positron emission tomography (PET)-imaging agent candidates of EGFR overexpressing tumors. J Med Chem 48:5337–5348. https://doi.org/10.1021/jm0580196
Miyazaki A, Miyake H, Fujisawa M (2016) Molecular mechanism mediating cytotoxic activity of axitinib in sunitinib-resistant human renal cell carcinoma cells. Clin Transl Oncol 18:893–900. https://doi.org/10.1007/s12094-015-1457-x
Moradpour Z, Barghi L (2019) Novel approaches for efficient delivery of tyrosine kinase inhibitors. J Pharm Pharm Sci 22:37–48. https://doi.org/10.18433/jpps29891
Morgillo F, Della Corte CM, Fasano M, Ciardiello F (2016) Mechanisms of resistance to EGFR-targeted drugs: lung cancer. ESMO Open 1:e000060. https://doi.org/10.1136/esmoopen-2016-000060
Mullard A (2018) FDA approves Eli Lilly’s baricitinib. Nat Rev Drug Discov 17:460–460. https://doi.org/10.1038/nrd.2018.112
O’Farrell A-M, Foran JM, Fiedler W et al (2003) An innovative phase I clinical study demonstrates inhibition of FLT3 phosphorylation by SU11248 in acute myeloid leukemia patients. Clin Cancer Res 9:5465–5476
Oda K, Matsuoka Y, Funahashi A, Kitano H (2005) A comprehensive pathway map of epidermal growth factor receptor signaling. Mol Syst Biol. https://doi.org/10.1038/msb4100014
Offin M, Liu D, Drilon A (2018) Tumor-agnostic drug development. Am Soc Clin Oncol Educ B. https://doi.org/10.1200/EDBK_200831
Osherov N, Levitzki A (1994) Epidermal-growth-factor-dependent activation of the Src-family kinases. Eur J Biochem 225:1047–1053. https://doi.org/10.1111/j.1432-1033.1994.1047b.x
Osherov N, Gazit A, Gilon C, Levitzki A (1993) Selective inhibition of the epidermal growth factor and HER2/neu receptors by tyrphostins. J Biol Chem 268:11134–11142. https://doi.org/10.1016/S0021-9258(18)82102-0
Ou X, Liu Y, Lei X et al (2020) Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun 11:1620. https://doi.org/10.1038/s41467-020-15562-9
Paez JG, Jänne PA, Lee JC et al (2004) EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science (80-) 304:1497–1500. https://doi.org/10.1126/science.1099314
Pennell NA, Lynch TJ (2009) Combined inhibition of the VEGFR and EGFR signaling pathways in the treatment of NSCLC. Oncologist 14:399–411. https://doi.org/10.1634/theoncologist.2008-0276
Planchard D, Popat S, Kerr K et al (2019) Correction to: “metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up.” Ann Oncol 30:863–870. https://doi.org/10.1093/annonc/mdy474
Pottier C, Wheatherspoon A, Roncarati P et al (2015) The importance of the tumor microenvironment in the therapeutic management of cancer. Expert Rev Anticancer Ther 15:943–954. https://doi.org/10.1586/14737140.2015.1059279
Pottier C, Fresnais M, Gilon M, Jérusalem G, Longuespée R, Sounni NE (2020) Tyrosine kinase inhibitors in cancer: breakthrough and challenges of targeted therapy. Cancers 12(3):731. https://doi.org/10.3390/cancers12030731
Prete A, Lo AS, Sadow PM et al (2018) Pericytes elicit resistance to vemurafenib and sorafenib therapy in thyroid carcinoma via the TSP-1/TGFβ1 Axis. Clin Cancer Res 24:6078–6097. https://doi.org/10.1158/1078-0432.CCR-18-0693
Rabindran SK, Discafani CM, Rosfjord EC et al (2004) Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res 64:3958–3965. https://doi.org/10.1158/0008-5472.CAN-03-2868
Rankin E, Giaccia A (2016) The receptor tyrosine kinase AXL in cancer progression. Cancers (basel) 8:103. https://doi.org/10.3390/cancers8110103
Roberts KG, Odell AF, Byrnes EM et al (2007) Resistance to c-KIT kinase inhibitors conferred by V654A mutation. Mol Cancer Ther 6:1159–1166. https://doi.org/10.1158/1535-7163.MCT-06-0641
Robinson DR, Wu Y-M, Lin S-F (2000) The protein tyrosine kinase family of the human genome. Oncogene 19:5548–5557. https://doi.org/10.1038/sj.onc.1203957
Roskoski R (2019) Properties of FDA-approved small molecule protein kinase inhibitors. Pharmacol Res 144:19–50. https://doi.org/10.1016/j.phrs.2019.03.006
Schlessinger J (2000) Cell signaling by receptor tyrosine kinases. Cell 103:211–225. https://doi.org/10.1016/S0092-8674(00)00114-8
Sequist LV, Yang JC-H, Yamamoto N et al (2013) Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 31:3327–3334. https://doi.org/10.1200/JCO.2012.44.2806
Shaul M, Abourbeh G, Jacobson O et al (2004) Novel iodine-124 labeled EGFR inhibitors as potential PET agents for molecular imaging in cancer. Bioorg Med Chem 12:3421–3429. https://doi.org/10.1016/j.bmc.2004.04.044
Shechter Y, Yaish P, Chorev M et al (1989) Inhibition of insulin-dependent lipogenesis and anti-lipolysis by protein tyrosine kinase inhibitors. EMBO J 8:1671–1676. https://doi.org/10.1002/j.1460-2075.1989.tb03558.x
Sims AC, Baric RS, Yount B et al (2005) Severe acute respiratory syndrome coronavirus infection of human ciliated airway epithelia: role of ciliated cells in viral spread in the conducting airways of the lungs. J Virol 79:15511–15524. https://doi.org/10.1128/JVI.79.24.15511-15524.2005
Smaill JB, Palmer BD, Rewcastle GW et al (1999) Tyrosine kinase inhibitors. 15. 4-(Phenylamino)quinazoline and 4-(Phenylamino)pyrido[d ]pyrimidine acrylamides as irreversible inhibitors of the ATP binding site of the epidermal growth factor receptor. J Med Chem 42:1803–1815. https://doi.org/10.1021/jm9806603
Smaill JB, Rewcastle GW, Loo JA et al (2000) Tyrosine kinase inhibitors. 17. irreversible inhibitors of the epidermal growth factor receptor: 4-(Phenylamino)quinazoline- and 4-(Phenylamino)pyrido[3,2- d ]pyrimidine-6-acrylamides bearing additional solubilizing functions. J Med Chem 43:1380–1397. https://doi.org/10.1021/jm990482t
Small BA, Dressel SA, Lawrence CW et al (2001) CD8+ T cell–mediated injury in vivo progresses in the absence of effector T cells. J Exp Med 194:1835–1846. https://doi.org/10.1084/jem.194.12.1835
Straussman R, Morikawa T, Shee K et al (2012) Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature 487:500–504. https://doi.org/10.1038/nature11183
Strawn LM, McMahon G, App H et al (1996) Flk-1 as a target for tumor growth inhibition. Cancer Res 56:3540–3545
Sun L, Tran N, Tang F et al (1998) Synthesis and biological evaluations of 3-substituted indolin-2-ones: a novel class of tyrosine kinase inhibitors that exhibit selectivity toward particular receptor tyrosine kinases. J Med Chem 41:2588–2603. https://doi.org/10.1021/jm980123i
Tan H-Y, Wang N, Lam W et al (2018) Targeting tumour microenvironment by tyrosine kinase inhibitor. Mol Cancer 17:43. https://doi.org/10.1186/s12943-018-0800-6
Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C (2020) Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol 11:1708. https://doi.org/10.3389/fimmu.2020.01708
Thobe BM et al (2006) Src family kinases regulate p38 MAPK-mediated IL-6 production in Kupffer cells following hypoxia. Am J Physiol Cell Physiol. https://doi.org/10.1152/ajpcell.00076.2006
Tolcher AW, Peng W, Calvo E (2018) Rational approaches for combination therapy strategies targeting the MAP kinase pathway in solid tumors. Mol Cancer Ther 17:3–16. https://doi.org/10.1158/1535-7163.MCT-17-0349
Tsou H-R, Mamuya N, Johnson BD et al (2001) 6-Substituted-4-(3-bromophenylamino)quinazolines as putative irreversible inhibitors of the epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor (HER-2) tyrosine kinases with enhanced antitumor activity. J Med Chem 44:2719–2734. https://doi.org/10.1021/jm0005555
Umezawa H, Imoto M, Sawa T, Isshiki K, Matsuda N, Uchida T, Iinuma H, Hamada M, Takeuchi T, Studies on a new Epidermal Growth Factor-Receptor Kinase Inhibitor, Erbstatin, produced by MH435-hF3, The Journal of Antibiotics, 1986, Volume 39, Issue 1, Pages 170–173, Released on J-STAGE April 19, 2006, Online ISSN 1881-1469, Print ISSN 0021-8820. https://doi.org/10.7164/antibiotics.39.170. https://www.jstage.jst.go.jp/article/antibiotics1968/39/1/39_1_170/_article/-char/en
Vincent PW, Bridges AJ, Dykes DJ et al (2000) Anticancer efficacy of the irreversible EGFr tyrosine kinase inhibitor PD 0169414 against human tumor xenografts. Cancer Chemother Pharmacol 45:231–238. https://doi.org/10.1007/s002800050034
Vogel A, Cervantes A, Chau I et al (2019) Correction to: “hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up.” Ann Oncol 30:871–873. https://doi.org/10.1093/annonc/mdy510
Wakeling A (2002) Epidermal growth factor receptor tyrosine kinase inhibitors. Curr Opin Pharmacol 2:382–387. https://doi.org/10.1016/S1471-4892(02)00183-2
Walls AC, Park Y-J, Tortorici MA et al (2020) Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 181:281-292.e6. https://doi.org/10.1016/j.cell.2020.02.058
Wang R, Li Y, Gong P et al (2018) Arsenic trioxide and sorafenib induce synthetic lethality of FLT3-ITD acute myeloid leukemia cells. Mol Cancer Ther 17:1871–1880. https://doi.org/10.1158/1535-7163.MCT-17-0298
Wang S, Qiu Z, Hou Y et al (2021) AXL is a candidate receptor for SARS-CoV-2 that promotes infection of pulmonary and bronchial epithelial cells. Cell Res 31:126–140. https://doi.org/10.1038/s41422-020-00460-y
Ward WHJ, Cook PN, Slater AM et al (1994) Epidermal growth factor receptor tyrosine kinase. Biochem Pharmacol 48:659–666. https://doi.org/10.1016/0006-2952(94)90042-6
Wong CK, Lam CWK, Wu AKL et al (2004) Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol 136:95–103. https://doi.org/10.1111/j.1365-2249.2004.02415.x
Wu Y-L, Cheng Y, Zhou X et al (2017) Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol 18:1454–1466. https://doi.org/10.1016/S1470-2045(17)30608-3
Wu Y, Kang L, Guo Z, Liu J, Liu M, Liang W (2022) Incubation period of COVID-19 caused by unique SARS-CoV-2 strains: a systematic review and Meta-analysis. JAMA Netw Open 5(8):e2228008. https://doi.org/10.1001/jamanetworkopen.2022.28008
Xiong Y, Liu Y, Cao L et al (2020) Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg Microbes Infect 9:761–770. https://doi.org/10.1080/22221751.2020.1747363
Yamaoka T, Kusumoto S, Ando K et al (2018) Receptor tyrosine kinase-targeted cancer therapy. Int J Mol Sci 19:3491. https://doi.org/10.3390/ijms19113491
Yang Z, Tam KY (2018) Combination strategies using EGFR-TKi in NSCLC therapy: learning from the gap between pre-clinical results and clinical outcomes. Int J Biol Sci 14:204–216. https://doi.org/10.7150/ijbs.22955
Yang L, Sun X, Ye Y et al (2019) p38α mitogen-activated protein kinase is a druggable target in pancreatic adenocarcinoma. Front Oncol. https://doi.org/10.3389/fonc.2019.01294
Yoshikawa T, Hill T, Li K et al (2009) Severe Acute respiratory syndrome (SARS) coronavirus-induced lung epithelial cytokines exacerbate SARS pathogenesis by modulating intrinsic functions of monocyte-derived macrophages and dendritic cells. J Virol 83:3039–3048. https://doi.org/10.1128/JVI.01792-08
Yu JSL, Cui W (2016) Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development 143:3050–3060. https://doi.org/10.1242/dev.137075
Yu S, Quinn D, Dorff T (2016) Clinical use of cabozantinib in the treatment of advanced kidney cancer: efficacy, safety, and patient selection. Onco Targets Ther 9:5825–5837. https://doi.org/10.2147/OTT.S97397
Zhang N, Liang C, Song W et al (2019) Antitumor activity of histone deacetylase inhibitor chidamide alone or in combination with epidermal growth factor receptor tyrosine kinase inhibitor icotinib in NSCLC. J Cancer 10:1275–1287. https://doi.org/10.7150/jca.28570
Zhang R, Wang X, Ni L et al (2020a) COVID-19: melatonin as a potential adjuvant treatment. Life Sci 250:117583. https://doi.org/10.1016/j.lfs.2020.117583
Zhang J, Litvinova M, Wang W et al (2020b) Evolving epidemiology and transmission dynamics of coronavirus disease 2019 outside Hubei province, China: a descriptive and modelling study. Lancet Infect Dis 20:793–802. https://doi.org/10.1016/S1473-3099(20)30230-9
Acknowledgements
The authors would like to thank the management of SRM Institute of Science and Technology for the infrastructural support.
Funding
This review article received no funding.
Author information
Authors and Affiliations
Contributions
The authors JN and TT were involved in the conceptualization and writing of the manuscript. The content of the images was prepared by TT and designed by JN. The contents were reviewed, edited, and formatted by AG, SM, MKK and JA. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by Yusuf Akhter.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Narayanan, J., Tamilanban, T., Kumar, P.S. et al. Role and mechanistic actions of protein kinase inhibitors as an effective drug target for cancer and COVID. Arch Microbiol 205, 238 (2023). https://doi.org/10.1007/s00203-023-03559-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-023-03559-z