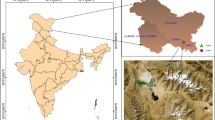
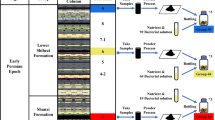
Abstract
The inappropriate disposal of toxic compounds generated by industrial activity has been impacting the environment considerably. Microbial communities inhabiting contaminated sites may represent interesting ecological alternatives for the decontamination of environments. The present work aimed to investigate the fungal diversity and its functionality contained in stream sediments with industrial waste contaminated with heavy metals by using metagenomic approach. A total of 12 fungal orders were retrieved from datasets and, at phylum level, Ascomycota was the most abundant, followed by Basidiomycota, Chytridiomycota and Blastocladiomycota. Higher abundance of sequences was encountered within the less contaminated site, while the lower abundance was found in the sample with the higher contamination with lead. Gene sequences related to DNA repair and heavy metals biosorption processes were found in the four samples analyzed. The genera Aspergillus and Chaetomium, and Saccharomycetales order were highly present within all samples, showing their potential to be used for bioremediation studies. The present work demonstrated the importance of using the metagenomic approach to understand the dynamics and the possible metabolic pathways associated with fungal communities related to environmental samples containing heavy metals, as well as evidenced the importance of improving culturomics techniques for isolating strains with potential application in bioremediation processes of environments contaminated with heavy metals.





Similar content being viewed by others
References
Abdel Azeen AM, El-Morsy EM, Nour El-Dein MM, Rashad HM (2015) Occurrence and diversity of mycobiota in heavy metal contaminated sediments of Mediterranean coastal lagoon El_Manzala, Egypt. Mycosphere 6:228–240
Aguiar JE, Marins RV, Almeida MD (2007) Comparação de metodologias de digestão de sedimentos marinhos para caracterização da geoquímica de metais-traço na plataforma continental Nordeste oriental brasileira. Geochim Bras 21:304–323
Ahmad I, Zafar S, Ahmad F (2005) Heavy Metal Biosorption potential of Aspergillus and Rhizopus sp. isolated from Wastewater treated soil. J Appl Sci Environ Manag 9:123–126
Akhtar S, Mahmood-ul-Hassan M, Ahmad R, Suthor V, Yasin M (2013) Metal tolerance potential of filamentous fungi isolated from soils irrigated with untreated municipal effluent. Soil Environ 32:55–62
Andrews S (2010) FastQC: a quality control tool for high throughput sequence data. http://www.bioinformatics.babraham.ac.uk/projects/fastqc
Baldrian P (2003) Interactions of heavy metals with white-rot fungi. Enzyme Microb Technol 32:78–91
Barone R, De Santi C, Esposito FP, Tedesco P, Galati F, Visone M, Di Scala A, De Pascale D (2014) Metagenomics, a valuable tool for enzymes and bioactive compounds discovery. Front Mar Sci 04:1–6
Beale DJ, Karpe AV, Jadhav S, Muster TH, Palombo EA (2015) Omics-based approaches and their use in the assessment of microbial-influenced corrosion of metals. Corros Rev 34:1–14
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120
Chen JP (2012) Decontamination of heavy metals: processes mechanisms and applications. CRC Press
Costa PS, Reis MP, Ávila MP, Leite LR, de Araújo FMG, Salim ACM, Oliveira G, Barbosa F, Chartone-Souza E, Nascimento AMA (2015) Metagenome of a microbial community inhabiting a metal-rich tropical stream sediment. PLoS ONE 10:1–21
Dhankhar R, Hooda A (2011) Fungal biosorption—an alternative to meet the challenges of heavy metal pollution in aqueous solutions. Environ Technol 32:467–491
Dopson M, Baker-Austin C, Koppineedi PR, Bond PL (2003) Growth in sulfidic mineral environments: metal resistance mechanisms in acidophilic micro-organisms. Microbiology 149:1959–1970
Feng G, Xie T, Wang X, Bai J, Tang L, Zhao H, Wei W, Wang M, Zhao Y (2018) Metagenomic analysis of microbial community and function involved in cd-contaminated soil. BMC Microbiol 18:1–13
Fidalgo CIA (2011) Heavy metal resistance in extremophilic yeasts a molecular and physiological approach. Dissertation, Universidade de Lisboa
Georg RC, Gomes SL (2007) Transcriptome analysis in response to heat shock and cadmium in the aquatic fungus Blastocladiella emersonii. Eukaryot Cell 6:1053–1062
Hammer Ø, Harper DAT, Ryan PD (2001) Past: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:4–9
Haruma T, Yamaji K, Masuya H, Hanyu K (2018) Root endophytic Chaetomium cupreum promotes plant growth and detoxifies aluminum in Miscanthus sinensis Andersson growing at the acidic mine site. Plant Species Biol 33:109–122
Hemme CL, Deng Y, Gentry TJ, Fields MW, Wu L, Barua S, Barry K, Tringe SG, Watson DB, He Z, Hazen TC, Tiedje JM, Rubin EM, Zhou J (2010) Metagenomic insights into evolution of a heavy metal-contaminated groundwater microbial community. ISME J 4:660–672
Hiraoka S, Yang CC, WaSaki W (2016) Metagenomics and bioinformatics in microbial ecology: current status and beyond. Microbes Environ 31:204–212
Iram S, Ahmad I, Javed B, Yaqoob S, Akhtar K, Kazmi MR, Zaman BU (2009) Fungal tolerance to heavy metals. Pak J Bot 41:2583–2594
Iram S, Parveen K, Usman J, Nasir K, Akhtar N, Arou S, Ahmad I (2012) Heavy metal tolerance of filamentous fungal strains isolated from soil irrigated with industrial wastewater. Biologija 58:107–116
Iskandar NL, Zainudin NAIM, Tan SG (2011) Tolerance and biosorption of copper (Cu) and lead (Pb) by filamentous fungi isolated from a freshwater ecosystem. J Environ Sci 23:824–830
Islam MS, Saha AK, Mosaddeque HQM, Amin MR, Islam MM (2008) In vitro studies on the reaction of fungi Trichoderma to different herbicides used in tea plantation. Int J Sustain Crop Prod 3:27–30
Jerônimo GH, de Jesus AL, Marano AV, James TY, de Souza JI, Rocha SCO, Pires-Zottarelli CLA (2015) Diversidade de blastocladiomycota e chytridiomycota do parque estadual da ilha do cardoso, cananéia, SP, Brasil. Hoehnea 42:135–163
Jia T, Wang R, Fan X, Chai B (2018) A Comparative study of fungal community structure, diversity and richness between the soil and the phyllosphere of native grass species in a Copper Tailings Dam in Shanxi Province China. Appl Sci 8:1297. https://doi.org/10.3390/app8081297
Joshi BH (2014) Evaluation and characterization of heavy metal resistant fungi for their prospects in bioremediation. J Environ Res Dev 8:876–882
Khamesy SJ, Hamidian AH, Atghia O (2016) Identification of the fungi absorbing heavy metals isolated from waste deposits of zinc factories, Zanjan province, Iran. Mycol Iran 3:65–73
Lehembre F, Doillon D, David E, Perotto S, Baude J, Foulon J, Harfouche L, Vallon L, Poulain J, Da Silva C, Wincher P, Oger-Desfeux C, Richaud P, Colpaert JV, Chalot M, Fraissinet-Tachet L, Blaudez D, Marmeisse R (2013) Soil metatranscriptomics for mining eukaryotic heavy metal resistance genes. Environ Microbiol 5:1–37
Magurran AF (2004) Measuring biological diversity. Blackwell, Oxford
Meyer F, Paarman D, D’Souza M, Olson R, Glass EM, Kubal M, Paczian T, Rodriguez A, Stevens R, Wilke A, Wilkening J, Edwards RA (2008) The metagenomics RAST server—a public resource for the automatic phylogenetic and functional analysis of metagenomes. BMC Bioinform 9:386
Mosier AC, Miller CS, Frischkorn KR, Ohm RA, Li Z, LaButti K, Lapidus A, Lipzen A, Chen C, Johnson J, Lindquist EA, Pan C, Hettich RL, Grigoriev IV, Singer SW, Banfield JF (2016) Fungi contribute critical but spatially varying roles in nitrogen and carbon cycling in acid mine drainage. Front Microbiol 7:1–18
Nogueira IS, Nabout JC, Oliveira JE, Silva KD (2008) Diversidade (alfa, beta e gama) da comunidade fitoplanctônica de quatro lagos artificiais urbanos do município de Goiânia, GO. Hoehnea 35:219–233
Nurk S, Meleshko D, Korobeynikov A, Pevzner PA (2017) metaSPAdes: a new versatile metagenomic assembler. Genome Res 27:824–834
Oladipo OG, Awotoye OO, Olayinka A, Ezeokoli OT, Maboeta MS, Bezuidenhout CC (2016) Heavy metal tolerance potential of Aspergillus strains isolated from mining sites. Biorem J 20:287–297
Page AJ, De Silva N, Hunt M, Quail MA, Parkhill J, Harris SR, Otto TD, Keane JA (2016) Robust high-throughput prokaryote de novo assembly and improvement pipeline for Illumina data. Microb Genom. https://doi.org/10.1099/mgen.0.000083
Pfister CA, Meyer F, Antonopoulos DA (2010) Metagenomic profiling of a microbial assemblage associated with the California mussel: a node in networks of carbon and nitrogen cycling. PLoS ONE 5:e105180
Pócsi I (2011) Toxic metal/metalloid tolerance in fungi—a biotechnology-oriented approach. In: Banfalvi G (ed) Cellular effects of heavy metals. Springer, Dordrecht, pp 31–58
Pryszcz LP, Gabaldón T (2016) Redundans: an assembly pipeline for highly heterozygous genomes. Nucleic Acids Res 44:e113
Qayyum S, Khan I, Maqbool F, Zhao Y, Gu Q, Peng G (2016) Isolation and characterization of heavy metal resistant fungal isolates from industrial soil in China. Pakistan J Zool 48:1241–1247
Rasool A, Irum S (2014) Toxic metal effect on filamentous fungi isolated from the contaminated soil of Multan and Gujranwala. J Bioresour Manag 1:38–51
Tsai SL, Singh S, Chen W (2009) Arsenic metabolism by microbes in nature and the impact on arsenic remediation. Curr Opin Biotechnol 20:659–667
USEPA (1994) Method 3015, Microwave assisted acid digestion of aqueous samples and extracts. USEPA, Washington
Viti C, Marchi E, Decorosi F, Giovannetti L (2014) Molecular mechanisms of Cr (VI) resistance in bacteria and fungi. FEMS Microbiol Rev 38:633–659
Woldeamanuale TB (2017) Isolation, screening and identification of cadmium tolerant fungi and their removal potential. J Forensic Sci Criminal Investig 5:555–656
World Health Organisation WHO (1989) Report of 33rd meeting Joint FAO/WHO Joint Expert committee on food additives Toxicological evaluation of certain food additives and contaminants No. 24 International Programme on Chemical Safety WHO Geneva
Xavier LC, Costa PES, Hissa DC, Melo VMM, Falcão RM, Balbino VQ, Mendonça LAR, Lima MGS, Coutinho HDM, Verde LCL (2019) Evaluation of the microbial diversity and heavy metal resistance genes of a microbial community on contaminated environment. Appl Geochem 105:1–6
Yin H, Niu J, Ren Y, Cong J, Zhang X, Fan F, Xiao Y, Zhang X, Deng J, Xie M, He Z, Zhou J, Liang Y, Liu X (2015) An integrated insight into the response of sedimentary microbial communities to heavy metal contamination. Sci Rep 5:1–12
Younus H (2018) Therapeutic potentials of superoxide dismutase. Int J Health Sci 12:88–93
Zhang X, Niu J, Liang Y, Liu X, Yin H (2016) Metagenome-scale analysis yields insights into the structure and function of microbial communities in a copper bioleaching heap. BMC Genet 17:4–12
Zhao D, Li T, Shen M, Wang J, Zhao Z (2015) Diverse strategies conferring extreme cadmium (Cd) tolerance in the dark septate endophyte (DSE), Exophiala pisciphila: evidence from RNA-seq data. Microbiol Res 170:27–35
Acknowledgements
We thank FUNCAP (Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico, Process number DCR-0024-01438.01.00/12) for their financial support. L. C. L. Verde was supported by the FUNCAP. We also thank to the CEGENBIO (Central de Genômica e Bioinformática do NPDM/UFC), by the genomic analysis.
Funding
Funcap - CEARENSE FOUNDATION FOR SUPPORT TO SCIENTIFIC AND TECHNOLOGICAL DEVELOPMENT (DCR-0024-01438.01.00/12).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

203_2022_2860_MOESM2_ESM.jpg
Supp 2 PCA plot illustrating the influence of variables on the alpha-diversity of the different sampled points (JPG 32 KB)
Rights and permissions
About this article
Cite this article
Passarini, M.R.Z., Ottoni, J.R., Costa, P.E.d. et al. Fungal community diversity of heavy metal contaminated soils revealed by metagenomics. Arch Microbiol 204, 255 (2022). https://doi.org/10.1007/s00203-022-02860-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-02860-7