Abstract
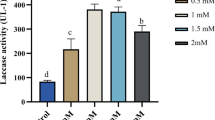
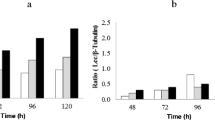
The effects of Cu2+ on the activity and expression of laccase were investigated in seven different strains of Pycnoporus coccineus collected from different regions in Korea. Cu2+ was toxic to mycelial growth at concentrations greater than 0.5 mM CuSO4 and showed complete growth inhibition at 1 mM in the liquid culture. However, Cu2+ significantly upregulated the extracellular laccase activity at 0.2 mM in five strains of P. coccineus, IUM4209, IUM0032, IUM0450, IUM0470, and IUM4093, whereas two strains, IUM0253 and IUM0049, did not respond to Cu2+, despite being closely related to the other five strains. Subsequent RT-PCR analysis also showed that the laccase mRNA was highly expressed only in the former five strains in the presence of Cu2+. Taken together, these results indicate that Cu2+ regulates expression of the laccase gene in a strain-dependent manner. The five strains commonly produced a single predominant laccase protein with a molecular weight of 68 kDa. Peptide sequencing revealed that the laccase was a homolog of Lcc1 of P. coccineus, which was isolated in China. The Cu2+-induced culture supernatants exhibited high degradation of polycyclic aromatic hydrocarbons, indicating that the 68-kDa laccase is the primary extracellular degradative enzyme in P. coccineus.





Similar content being viewed by others
References
Barr DP, Aust AD (1994) Mechanisms white rot fungi use to degrade pollutants. Environ Sci Technol 28:78A–87A
Castanera R, Pérez G, Omarini A, Alfaro M, Pisabarro AG, Faraco V, Amore A, Ramírez L (2012) Transcriptional and enzymatic profiling of Pleurotus ostreatus laccase genes in submerged and solid-state fermentation cultures. Appl Environ Microbiol 78:4037–4045
Cerniglia CE (1992) Biodegradation of polycyclic aromatic hydrocarbons. Biodegradation 3:351–368
Dantan-Gonzalez E, Vite-Vallejo O, Martinez-Anaya C, Mendez-Sanchez M, Gonzalez MC, Palomares LA, Folch-Mallol J (2008) Production of two novel laccase isoforms by a thermotolerant strain of Pycnoporus sanguineus isolated from an oil-polluted tropical habitat. Int Microbiol 11:163–169
Dekker RF, Barbosa AM, Giese EC, Godoy SD, Covizzi LG (2007) Influence of nutrients on enhancing laccase production by Botryosphaeria rhodina MAMB-05. Int Microbiol 10:177–185
Dos Santos AZ, Cândido Neto JM, Tavares CR, da Costa SM (2004) Screening of filamentous fungi for the decolorization of a commercial reactive dye. J Basic Microbiol 44:288–295
Eggert C, Temp U, Dean JF, Eriksson KE (1995) Laccase-mediated formation of the phenoxazinone derivative, cinnabarinic acid. FEBS Lett 376:202–206
Eggert C, Temp U, Eriksson KE (1996) The ligninolytic system of the white rot fungus Pycnoporus cinnabarinus: purification and characterization of the laccase. Appl Environ Microbiol 62:1151–1158
Eugenio ME, Carbajo JM, Martín JA, González AE, Villar JC (2009) Laccase production by Pycnoporus sanguineus under different culture conditions. J Basic Microbiol 49:433–440
Galhaup C, Haltrich D (2001) Enhanced formation of laccase activity by the white-rot fungus Trametes pubescens in the presence of copper. Appl Microbiol Biotechnol 56:225–232
Garcia TA, Santiago MF, Ulhoa CJ (2006) Properties of laccases produced by Pycnoporus sanguineus induced by 2,5-xylidine. Biotechnol Lett 28:633–636
Geng X, Li K (2002) Degradation of non-phenolic lignin by the white-rot fungus Pycnoporus cinnabarinus. Appl Microbiol Biotechnol 60:342–346
Hartikainen ES, Lankinen P, Rajasärkkä J, Koponen H, Virta M, Hatakka A, Kähkönen MA (2012) Impact of copper and zinc on the growth of saprotrophic fungi and the production of extracellular enzymes. Boreal Environ Res 17:210–218
Hoshida H, Nakao M, Kanazawa H, Kubo K, Hakukawa T, Morimasa K, Akada R, Nishizawa Y (2001) Isolation of five laccase gene sequences from the white-rot fungus Trametes sanguinea by PCR, and cloning, characterization and expression of the laccase cDNA in yeasts. J Biosci Bioeng 92:372–380
Jaouani A, Guillen F, Penninckx MJ, Martinez A, Martinez MJ (2005) Role of Pycnoporus coccineus laccase in the degradation of aromatic compounds in olive oil mill wastewater. Enzyme Microb Technol 36:478–486
Le Quéré A, Eriksen KA, Rajashekar B, Schützendübel A, Canbäck B, Johansson T, Tunlid A (2006) Screening for rapidly evolving genes in the ectomycorrhizal fungus Paxillus involutus using cDNA microarrays. Mol Ecol 15:535–550
Lim Y, Ryu JS, Shi S, Noh W, Kim EM, Le QV, Lee HS, Ro HS (2008) Isolation of bacteria associated with the king oyster mushroom, Pleurotus eryngii. Mycobiology 36:13–18
Lisova ZA, Lisov AV, Leontievsky AA (2010) Two laccase isoforms of the basidiomycete Cerrena unicolor VKMF-3196. Induction, isolation and properties. J Basic Microbiol 50:72–82
Lomascolo A, Uzan-Boukhris E, Herpoel-Gimbert I, Sigoillot JC, Lesage-Meessen L (2011) Peculiarities of Pycnoporus species for applications in biotechnology. Appl Microbiol Biotechnol 92:1129–1149
Madhavi V, Lele SS (2009) Laccase: properties and applications. BioResources 4:1694–1717
Majcherczyk A, Johannes C, Hüttermann A (1998) Oxidation of polycyclic aromatic hydrocarbons (PAH) by laccase of Trametes Versicolor. Enzyme Microb Technol 22:335–341
Morgenstern I, Robertson DL, Hibbett DS (2010) Characterization of three mnp genes of Fomitiporia mediterranea and report of additional class II peroxidases in the order Hymenochaetales. Appl Environ Microbiol 76:6431–6440
Muller M, Shi C (2001) Laccase for denim processing. AATCC Rev 2001:4–5
Niku-Paavola ML, Karhunen E, Salola P, Raunio V (1988) Ligninolytic enzymes of the white-rot fungus Phlebia radiata. Biochem J 254:877–884
Oda Y, Adachi K, Aita I, Ito M, Aso Y, Igarashi H (1991) Purification and properties of laccase excreted by Pycnoporus coccineus. Agric Biol Chem 55:1393–1396
Pointing SB, Pelling AL, Smith JD, Hyde KD, Reddy CA (2005) Screening of basidiomycetes and xylariouceaous fungi for lignin peroxidase and laccase gene-specific sequences. Mycol Res 109:115–124
Punnapayak H, Prasongsuk S, Messner K, Danmek K, Lotrakul P (2009) Polycyclic aromatic hydrocarbons (PAHs) degradation by laccase from a tropical white rot fungus Ganoderma lucidum. Afr J Biotechnol 8:5897–5900
Reid ID (1991) Biological pulping in paper manufacture. Trends Biotechnol 9:262–265
Ro HS, Kim SS, Ryu JS, Jeon CO, Lee TS, Lee HS (2007) Comparative studies on the diversity of the edible mushroom Pleurotus eryngii: iTS sequence analysis, RAPD fingerprinting, and physiological characteristics. Mycol Res 111:710–715
Rohr CO, Levin LN, Mentaberry AN, Wirth SA (2013) A first insight into Pycnoporus sanguineus BAFC 2126. PLoS One 8:e81033
Saparrat M, Balatti PA, Martínez MJ, Jurado M (2010) Differential regulation of laccase gene expression in Coriolopsis rigida LPSC No. 232. Fungal Biol 114:999–1006
Sato A, Watanabe T, Watanabe Y, Harazono K, Fukatsu T (2002) Screening for basidiomycetous fungi capable of degrading 2,7-dichlorodibenzo-p-dioxin. FEMS Microbiol Lett 213:213–217
Soares A, Jonasson K, Terrazas E, Guieysse B, Mattiasson B (2005) The ability of white-rot fungi to degrade the endocrine-disrupting compound nonylphenol. Appl Microbiol Biotechnol 66:719–725
Sullivan G, Henry ED (1971) Occurrence and distribution of phenoxazinone pigments in the genus pycnoporus. J Pharm Sci 60:1097–1098
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tong P, Hong Y, Xiao Y, Zhang M, Tu X, Cui T (2007) High production of laccase by a new basidiomycete, Trametes sp. Biotechnol Lett 29:295–301
Uzan E, Nousiainen P, Balland V, Sipila J, Piumi F, Navarro D, Asther M, Record E, Lomascolo A (2010) High redox potential laccases from the lignolytic fungi Pycnoporus coccineus and P. sanguineus suitable for white biotechnology: from gene cloning to enzyme characterization and applications. J Appl Microbiol 108:2199–2213
Van Aken B, Agathos SN (2002) Implication of manganese (III), oxalate, and oxygen in the degradation of nitroaromatic compounds by manganese peroxidase (MnP). Appl Microbiol Biotechnol 58:345–351
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press Inc, New York, pp 315–322
Acknowledgments
This study was supported by “The Genetic Evaluation of Important Biological Resources” grant funded by the National Institute of Biological Resources, Korea. JWP, BSH, and SK were supported by a scholarship from the BK 21 Plus Program, maintained by the Ministry of Education, Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Olaf Kniemeyer.
Rights and permissions
About this article
Cite this article
Park, JW., Kang, HW., Ha, BS. et al. Strain-dependent response to Cu2+ in the expression of laccase in Pycnoporus coccineus . Arch Microbiol 197, 589–596 (2015). https://doi.org/10.1007/s00203-015-1090-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-015-1090-7