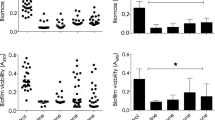
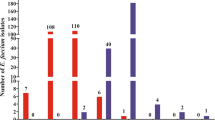
Abstract
Multi- and pan-antibiotic-resistant bacteria are a major health challenge in hospital settings. Furthermore, when susceptible bacteria establish surface-attached biofilm populations, they become recalcitrant to antimicrobial therapy. Therefore, there is a need for novel antimicrobials that are effective against multi-drug-resistant and surface-attached bacteria. A screen to identify prokaryote-derived antimicrobials from a panel of over 100 bacterial strains was performed. One compound isolated from Citrobacter freundii exhibited antimicrobial activity against a wide range of Gram-negative bacteria and was effective against biofilms. Random transposon mutagenesis was performed to find mutants unable to produce the antimicrobial compound. Transposons mapped to a bacteriocin gene located on a small plasmid capable of replication in Escherichia coli. The plasmid was sequenced and found to be highly similar to a previously described colicinogenic plasmid. Expression of the predicted bacteriocin immunity gene conferred bacteriocin immunity to E. coli. The predicted bacteriocin gene, colA-43864, expressed in E. coli was sufficient to generate anti-microbial activity, and purified recombinant ColA-43864 was highly effective in killing E. coli, Citrobacter species, and Klebsiella pneumoniae cells in a planktonic and biofilm state. This study suggests that bacteriocins can be an effective way to control surface-attached pathogenic bacteria.







Similar content being viewed by others
References
Anderson GG, O’Toole GA (2008) Innate and induced resistance mechanisms of bacterial biofilms. Curr Top Microbiol Immunol 322:85–105
Bush K (2010) Alarming beta-lactamase-mediated resistance in multidrug-resistant Enterobacteriaceae. Curr Opin Microbiol 13:558–564
Cascales E et al (2007) Colicin biology. Microbiol Mol Biol Rev 71:158–229
Chai T, Wu V, Foulds J (1982) Colicin A receptor: role of two Escherichia coli outer membrane proteins (OmpF protein and btuB gene product) and lipopolysaccharide. J Bacteriol 151:983–988
Croal LR, Jiao Y, Newman DK (2007) The fox operon from Rhodobacter strain SW2 promotes phototrophic Fe(II) oxidation in Rhodobacter capsulatus SB1003. J Bacteriol 189:1774–1782
Crozel V, Lazdunski C, Cavard D (1983) Localization of genes responsible for replication and immunity to colicin A on plasmid ColA-CA31. Mol Gen Genet 192:500–505
Dashiff A, Junka RA, Libera M, Kadouri DE (2011) Predation of human pathogens by the predatory bacteria Micavibrio aeruginosavorus and Bdellovibrio bacteriovorus. J Appl Microbiol 110:431–444
Davies JK, Reeves P (1975) Genetics of resistance to colicins in Escherichia coli K-12: cross-resistance among colicins of group A. J Bacteriol 123:102–117
Donlan RM (2001) Biofilm formation: a clinically relevant microbiological process. Clin Infect Dis 33:1387–1392
Donlan RM, Costerton JW (2002) Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15:167–193
Hancock V, Dahl M, Klemm P (2010) Probiotic Escherichia coli strain Nissle 1917 outcompetes intestinal pathogens during biofilm formation. J Med Microbiol 59:392–399
Hecht O, Zhang Y, Li C, Penfold CN, James R, Moore GR (2010) Characterisation of the interaction of colicin A with its co-receptor TolA. FEBS Lett 584:2249–2252
Hoiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O (2010) Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents 35:322–332
Janda MJ, Abbott SL (2006) The Enterobacteria, 2nd edn. American Society of Microbiology, Washington, DC
Kadouri D, O’Toole GA (2005) Susceptibility of biofilms to Bdellovibrio bacteriovorus attack. Appl Environ Microbiol 71:4044–4051
Kadouri D, Venzon NC, O’Toole GA (2007) Vulnerability of pathogenic biofilms to Micavibrio aeruginosavorus. Appl Environ Microbiol 73:605–614
Kreth J, Merritt J, Shi W, Qi F (2005) Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm. J Bacteriol 187:7193–7203
Kulasekara HD, Ventre I, Kulasekara BR, Lazdunski A, Filloux A, Lory S (2005) A novel two-component system controls the expression of Pseudomonas aeruginosa fimbrial cup genes. Mol Microbiol 55:368–380
Lewis K (2010) Persister cells. Annu Rev Microbiol 64:357–372
Mah TF, O’Toole GA (2001) Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol 9:34–39
Medina AA, Shanks RM, Kadouri DE (2008) Development of a novel system for isolating genes involved in predator-prey interactions using host independent derivatives of Bdellovibrio bacteriovorus 109J. BMC Microbiol 8:33
Merritt JH, Kadouri DE, O’Toole GA (2005) Growing and analyzing static biofilms. In: Current protocols in microbiology, pp 1B.1.1–1B.1.17
Morlon J, Cavard D, Lazdunski C (1982) Physical map of pColA-CA31, an amplifiable plasmid, and location of colicin A structural gene. Gene 17:317–321
Morlon J, Chartier M, Bidaud M, Lazdunski C (1988a) The complete nucleotide sequence of the colicinogenic plasmid ColA. High extent of homology with ColE1. Mol Gen Genet 211:231–243
Morlon J, Sherratt D, Lazdunski C (1988b) Identification of functional regions of the colicinogenic plasmid ColA. Mol Gen Genet 211:223–230
Nordmann P (1998) Trends in beta-lactam resistance among Enterobacteriaceae. Clin Infect Dis 27(Suppl 1):S100–S106
O’Toole GA, Kolter R (1998) Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol 30:295–304
Pillai DR, McGeer A, Low DE (2011) New Delhi metallo-beta-lactamase-1 in Enterobacteriaceae: emerging resistance. CMAJ 183:59–64
Schein SJ, Kagan BL, Finkelstein A (1978) Colicin K acts by forming voltage-dependent channels in phospholipid bilayer membranes. Nature 276:159–163
Shanks RM, Caiazza NC, Hinsa SM, Toutain CM, O’Toole GA (2006) Saccharomyces cerevisiae-based molecular tool kit for manipulation of genes from gram-negative bacteria. Appl Environ Microbiol 72:5027–5036
Shanks RM, Kadouri DE, MacEachran DP, O’Toole GA (2009) New yeast recombineering tools for bacteria. Plasmid 62:88–97
Stewart PS, Costerton JW (2001) Antibiotic resistance of bacteria in biofilms. Lancet 358:135–138
Tait K, Sutherland IW (2002) Antagonistic interactions amongst bacteriocin-producing enteric bacteria in dual species biofilms. J Appl Microbiol 93:345–352
Varenne S, Cavard D, Lazdunski C (1981) Biosynthesis and export of colicin A in Citrobacter freundii CA31. Eur J Biochem 116:615–620
Acknowledgments
The authors would like to thank Nicholas Stella for technical assistance and Dr. Yohei Doi, Division of Infectious Diseases, University of Pittsburgh School of Medicine for kindly providing bacterial strains. This work was supported by funding from the Foundation of UMDNJ faculty research grant to D.E.K., and NIH AI085570 and a Research to Prevent Blindness Career Development Award to R.M.Q.S. Additional support was provided by NIH grant EY08098 and the Eye and Ear Foundation of Pittsburgh.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Erko Stackebrandt.
Rights and permissions
About this article
Cite this article
Shanks, R.M.Q., Dashiff, A., Alster, J.S. et al. Isolation and identification of a bacteriocin with antibacterial and antibiofilm activity from Citrobacter freundii . Arch Microbiol 194, 575–587 (2012). https://doi.org/10.1007/s00203-012-0793-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-012-0793-2