Abstract
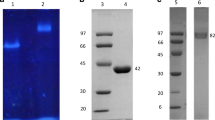
We screened for microorganisms able to use flavonoids as a carbon source; and one isolate, nominated Stilbella fimetaria SES201, was found to possess a disaccharide-specific hydrolase. It was a cell-bound ectoenzyme that was released to the medium during conidiogenesis. The enzyme was shown to cleave the flavonoid hesperidin (hesperetin 7-O-α-rhamnopyranosyl-β-glucopyranoside) into rutinose (α-rhamnopyranosyl-β-glucopyranose) and hesperetin. Since only intracellular traces of monoglycosidase activities (β-glucosidase, α-rhamnosidase) were produced, the disaccharidase α-rhamnosyl-β-glucosidase was the main system utilized by the microorganism for hesperidin hydrolysis. The enzyme was a glycoprotein with a molecular weight of 42224 Da and isoelectric point of 5.7. Even when maximum activity was found at 70°C, it was active at temperatures as low as 5°C, consistent with the psychrotolerant character of S. fimetaria. Substrate preference studies indicated that the enzyme exhibits high specificity toward 7-O-linked flavonoid β-rutinosides. It did not act on flavonoid 3-O-β-rutinoside and 7-O-β-neohesperidosides, neither monoglycosylated substrates. In an aqueous medium, the α-rhamnosyl-β-glucosidase was also able to transfer rutinose to other acceptors besides water, indicating its potential as biocatalyst for organic synthesis. The monoenzyme strategy of S. fimetaria SES201, as well as the enzyme substrate preference for 7-O-β-flavonoid rutinosides, is unique characteristics among the microbial flavonoid deglycosylation systems reported.







Similar content being viewed by others
References
Ahn YO, Mizutani M, Saino H, Sakata K (2004) Furcatin hydrolase from Viburnum furcatum Blume is a novel disaccharide-specific acuminosidase in glycosyl hydrolase family 1. J Biol Chem 279:23405–23414
Barbagallo RN, Spagna G, Palmeri R, Restuccia C, Giudici P (2004) Selection, characterization and comparison of β-glucosidase from mould and yeasts employable for enological applications. Enz Microb Technol 35:58–66
Baumgertel A, Grimm R, Eisenbeiß W, Kreis W (2003) Purification and characterization of a flavonol 3-O-β-heterodisaccharidase from the dried herb of Fagopyrum esculentum Moench. Phytochem 64:411–418
Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B (2009) The Carbohydrate-Active EnZymes database (CAZy): an expert resource for Glycogenomics. Nucleic Acids Res 37:D233–D238
Cassago A, Panepucci RA, Tortella Baião AM, Henrique-Silva F (2002) Cellophane based mini-prep method for DNA extraction from the filamentous fungus Trichoderma reesei. BMC Microbiol 2:14–18
Chuankhayan P, Hua Y, Svasti J, Sakdarat S, Sullivan PA, Ketudat Cairns JR (2005) Purification of an isoflavonoid 7-O-β-apiosyl-glucoside β-glycosidase and its substrates from Dalbergia nigrescens Kurz. Phytochem 66:1880–1889
Daiyasu H, Saino H, Tomoto H, Mizutani M, Sakata K, Toh H (2008) Computational and experimental analyses of furcatin hydrolase for substrate specificity studies of disaccharide-specific glycosidases. J Biochem 144:467–475
Genovés S, Gil JV, Vallés S, Casas JA, Manzanares P (2005) Assessment of the aromatic potential of palomino fino grape must using glycosidases. Am J Enol Vitic 56:188–191
Gholipour Y, Nonami H, Erra-Balsells R (2008) In situ analysis of plant tissue underivatized carbohydrates and on-probe enzymatic degraded starch by UV-carbon nanotube-assisted laser desorption/ionization TOF mass spectrometry. Anal Biochem 383:159–167
Grimaldi A, McLean H, Jiranek V (2000) Identification and partial characterization of glycosidic activities of commercial strains of the lactic acid bacterium Oenococcus oeni. Am J Enol Viticul 51:362–369
Hemingway KM, Alston MJ, Chappell CG, Taylor AJ (1999) Carbohydrate-flavour conjugates in wine. Carbohydr Polym 38:283–286
Henrissat B, Sulzenbacher G, Bourne Y (2008) Glycosyltransferases, glycoside hydrolases: surprise, surprise!. Curr Opin Struct Biol 18:527–533
Ibarz JM, Ferreira V, Hernández-Orte P, Loscos N, Cacho J (2006) Optimization and evaluation of a procedure for the gas chromatographic-mass spectrometric analysis of the aromas generated by fast acid hydrolysis of flavor precursors extracted from grapes. J Chromatogr A 1116:217–229
Jaworski A, Brückner H (2001) Sequences of polypeptide antibiotics stilboflavins, natural peptaibol libraries of the mold Stilbella flavipes. J Pept Scien 7:433–447
Kafanova TV, Busarova NG, Khudyakova YV, Isai SV (2008) Marine fungus Stilbella aciculosa as a potential producer of prostaglandins. Microbiology 77:451–454
Kato N, Suyama S, Shirokane M, Kato M, Kobayashi T, Tsukagoshi N (2002) Novel-glucosidase from Aspergillus nidulans with strong transglycosylation activity. Appl Environ Microbiol 68:1250–1256
Klose S, Tabatabai MA (2002) Response of glycosidases in soils to chloroform fumigation. Biol Fertil Soils 35:262–269
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lai LB, Gopalan V, Glew RH (1992) Continuous spectrophotometric assays for β-glucosidases acting on the plant glucosides L-picein and prunasin. Anal Biochem 2:365–369
Lehr NA, Meffert A, Antelo L, Sterner O, Anke H, Weber RWS (2006) Antiamoebins, myrocin B and the basis of antifungal antibiosis in the coprophilous fungus Stilbella erythrocephala (syn. S. fimetaria). FEMS Microbiol Ecol 55:105–112
Ma SJ, Mizutani M, Hiratake J, Hayashi K, Yagi K, Watanabe N, Sakata K (2001) Substrates specificity of β-primeverosidase, a key enzyme in aroma formation during oolong tea and black tea manufacturing. Biosci Biotechnol Biochem 65:2719–2729
Manthey JA, Grohmann K (1996) Concentrations of hesperidin and other orange peel flavonoids in citrus processing byproducts. J Agric Food Chem 44:811–814
Manzanares P, van den Broeck HC, de Graaff LH, Visser J (2001) Purification and characterization of two different α-l-rhamnosidases, RhaA and RhaB, from Aspergillus aculeatus. Appl Environ Microbiol 67:2230–2234
Martearena M, Daz M, Ellenrieder G (2007) Synthesis of rutinosides and rutinose by reverse hydrolysis catalyzed by fungal α-l-rhamnosidases. Biocatal Biotransf 26:177–185
Martínez MA, Delgado OD, Breccia JD, Baigorí MD, Siñeriz F (2002) Revision of the taxonomic position of the xylanolytic Bacillus sp. MIR32 reidentified as Bacillus halodurans and plasmid-mediated transformation of B. halodurans. Extremophiles 6:391–395
Mauludin R, Müller RH (2008) Hesperidin smart crystals: redispersibility and improved solubility properties. Pharmacogenetics/Pharmacogenomics Virtual J (http://www.aapsj.org) 10: S2
Miller GL (1959) Use of dinitrosalisylic acid (DNS) for determination of reducing sugars. Anal Chem 31:426–428
Mizutani M, Nakanishi H, Ema J, Ma SJ, Noguchi E, Inohara-Ochiai M, Fukuchi-Mizutani M, Nakao M, Sakata K (2002) Cloning of β-primeverosidase from tea leaves, a key enzyme in tea aroma formation. Plant Physiol 130:2164–2176
Møller HJ, Poulsen HJ (1996) Staining of Glycoproteins/Proteoglycans on SDS-Gels. In: Walker JM (ed) The protein protocols handbook. Humana Press, New York, pp 627–631
Monti D, Pišvejcová A, Křen V, Lama M, Riva S (2004) Generation of an α-l-rhamnosidase library and its application for the selective derhamnosylation of natural products. Biotechnol Bioeng 87:763–771
Monti D, Candido A, Cruz Silva MM, Křen V, Riva S, Danieli B (2005) Biocatalyzed generation of molecular diversity: selective modification of the saponin asiaticoside. Adv Synth Catal 347:1168–1174
Nakanishi F, Nagasawa Y, Kabaya Y, Sekimoto H, Shimomura K (2005) Characterization of lucidin formation in Rubia tinctorum L. Plant Physiol Biochem 43:921–928
Narikawa T, Shinoyama H, Fujii T (2000) A β-rutinosidase from Penicillium rugulosum IFO 7242 that is a peculiar flavonoid glycosidase. Biosci Biotechnol Biochem 64:1317–1319
Nonami H, Fukui S, Erra-Balsells R (1997) β-Carboline alkaloids as matrices for matrix-assisted ultraviolet laser desorption time-of flight mass spectrometry of proteins and sulfated oligosaccharides: a comparative study using phenylcarbonyl compounds, carbazoles and classical matrices. J Mass Spectrom 32:287–296
Oakley BR, Kirsch DR, Morris NR (1980) A simplified ultrasensitive stain for detecting proteins in polyacrylamide gels. Anal Biochem 105:361–363
Orrillo AG, Ledesma P, Delgado OD, Spagna G, Breccia JD (2007) Cold-active α-l-rhamnosidase from psychrotolerant bacteria isolated from a sub-Antarctic ecosystem. Enz Microb Technol 40:236–241
Polaina J, MacCabe AP (2007) Industrial enzymes. Function and applications. Springer, Netherlands
Promega (2008) Protocols and application guide. Promega Corporation, Madison, Wisconsin
Puder C, Wagner K, Vettermann R, Hauptmann R, Potterat O (2005) Terphenylquinone inhibitors of the Src protein tyrosine kinase from Stilbella fimetaria. J Nat Produc 68:323–326
Russell NJ (2000) Toward a molecular understanding of cold activity of enzymes from psychrophiles. Extremophiles 4:83–90
Sang-Joon L, Omori T, Kodama T (1990) Purification and some properties of rutinosidase from Arthrobacter sp. Kor J Appl Microbiol Biotech 18:360–367
Sarry JE, Gunata Z (2004) Plant and microbial glycoside hydrolases: volatile release from glycosidic aroma precursors. Food Chem 87:509–521
Schimel JP, Fahnestock J, Michaelson G, Mikan C, Ping C-L, Romanovsky VE, Welker J (2006) Cold-season production of CO2 in Arctic soils: can laboratory and field estimates be reconciled through a simple modeling approach? Arct Antarc Alp Res 38:249–256
Seifert KA (1985) A monograph of Stilbella and some allied Hyphomycetes. Stud Mycol 27:1–235
Shaw LJ, Morris P, Hooker JE (2006) Perception and modification of plant flavonoid signals by rhizosphere microorganisms. Environ Microbiol 8:1867–1880
Slapeta J, Moreira D, López-García P (2005) The extent of protist diversity: insights from molecular ecology of freshwater eukaryotes. Proc R Soc B 272:2073–2081
Spagna G, Barbagallo RN, Greco E, Manenti I, Pifferi P (2002) A mixture of purified glycosidases from Aspergillus niger for oenological application immobilized by inclusion in chitosan gels. Enz Microb Technol 30:80–89
Tsuruhami K, Mori S, Amarume S, Sarawatari S, Murata T, Hirakake J, Sakata K, Usui T (2006) Isolation and characterization of a β-primeverosidase-like enzyme from Penicillium multicolor. Biosci Biotechnol Biochem 70:691–698
Wang D, Kurasawa E, Yamaguchi Y, Kubota K, Kobayashi A (2001) Analysis of glycosidically bound aroma precursors in tea leaves. 2. Changes in glycoside contents and glycosidase activities in tea leaves during the black tea manufacturing process. J Agric Food Chem 49:1900–1903
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M, Gelfand D, Sninsky J, White T (eds) PCR protocols: a guide to methods and applications. Academic Press, Orlando, pp 315–322
Williams PJ, Strauss CR, Wilson B, Massy-Westropp RA (1982) Use of C18 reversed-phase liquid chromatography for the isolation of monoterpene glycosides and nor-isoprenoid precursors from grape juice and wines. J Chromatogr 235:471–480
Yamamoto S, Okada M, Usui T, Sakata K (2002) Isolation and characterization of a β-primeverosidase-like endo-manner β-glycosidase from Aspergillus fumigatus AP-20. Biosci Biotechnol Biochem 66:801–807
Yamamoto S, Okada M, Usui T, Sakata K, Toumoto A, Tsuruhami K (2006) Diglycosidase isolated from microorganisms. US Patent 7109014
Acknowledgments
This work was supported by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Universidad Nacional de La Pampa (UNLPam) and Agencia Nacional de Promoción Científica y Técnica (ANPCyT) of Argentina. The authors gratefully thank the contributions of Eduardo Piontelli, Jorge Oyhenart and Alejandra Martínez in strain identification, Martin Hedström for mass spectrometry analysis and María Rita Martearena, Elsa Scaroni and Mirta Daz for the generous gift of flavonoids. Finally, we are indebted to Maria Andersson for helpful suggestions and critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Erko Stackebrandt.
An erratum to this article can be found at http://dx.doi.org/10.1007/s00203-011-0709-6
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mazzaferro, L., Piñuel, L., Minig, M. et al. Extracellular monoenzyme deglycosylation system of 7-O-linked flavonoid β-rutinosides and its disaccharide transglycosylation activity from Stilbella fimetaria . Arch Microbiol 192, 383–393 (2010). https://doi.org/10.1007/s00203-010-0567-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-010-0567-7