Abstract
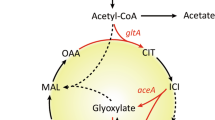
To elucidate the physiological adaptation of Escherichia coli due to cra gene knockout, a total of 3,911 gene expressions were investigated by DNA microarray for continuous culture. About 50 genes were differentially regulated for the cra mutant. TCA cycle and glyoxylate shunt were down-regulated, while pentose phosphate (PP) pathway and Entner Doudoroff (ED) pathway were up-regulated in the cra mutant. The glucose uptake rate and the acetate production rate were increased with less acetate consumption for the cra mutant. To identify the genes controlled by Cra protein, the Cra recognition weight matrix from foot-printing data was developed and used to scan the whole genome. Several new Cra-binding sites were found, and some of the result was consistent with the DNA microarray data. The ED pathway was active in the cra mutant; we constructed cra.edd double genes knockout mutant to block this pathway, where the acetate overflowed due to the down-regulation of aceA,B and icd gene expressions. Then we further constructed cra.edd.iclR triple genes knockout mutant to direct the carbon flow through the glyoxylate pathway. The cra.edd.iclR mutant showed the least acetate production, resulting in the highest cell yield together with the activation of the glycolysis pathway, but the glucose consumption rate could not be improved.





Similar content being viewed by others
References
Baba T, Ara T, Hasegawa M, Takai T, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H (2006) Construction of E. coli K12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol. doi:10.1038/msb4100050(E1-E11)
Berg OG, von Hippel H (1987) Selection of DNA binding sites by regulatory proteins. Statistical–mechanical theory and applications to operators and promoters. J Mol Biol 193:723–750
Cortay J-C, Negre D, Scarabel M, Ramseier TM, Vartak NB, Reizer J, Saier MH Jr, Cozoone A (1994) In vitro asymmetric binding of the pleiotropic regulatory protein, FruR, to the ace operator controlling glyoxalate shunt enzyme synthesis. J Biol Chem 269:14885–14891
Cunningham L, Gruer MJ, Guest JR (1997) Transcriptional regulation of the aconitase genes (acnA and acnB) of Escherichia coli. Microbiology 143:3795–3805
Datsenko KA, Wanner BL (2001) One step inactivation of chromosomal genes in Escherichia coli K-12 using PCR product. Proc Natl Acad Sci USA 97:6640–6645
Farmer WR, Liao JC (1997) Reduction of aerobic acetate production by Escherichia coli. Appl Environ Microbiol 63:3205–3210
Kabir MM, Shimizu K (2003) Gene expression patterns for metabolic pathway in pgi knockout Escherichia coli with and without phb genes based on RT-PCR. J Biotechnol 105:11–31
Keseler IM, Collado-Vides J, Gama-Castro S, Ingraham J, Paley S, Paulsen IT, Peralta-Gil M, Karp PD (2005) EcoCyc: a comprehensive database resource for Escherichia coli. Nucl Acid Res 33:D334–D337 (database issue)
Koebman BJ, Weaterhoff HW, Snoop JN, Nilsson D, Jensen PR (2002) The glycolytic flux in Escherichia coli is controlled by the demand for ATP. J Bacteriol 184:3909–3916
Liu X, Wulf PD (2004) Probing the ArcA-P modulon of Escherichia coli by whole genome transcriptional analysis and sequence recognition profiling. J Biol Chem 279:12588–12597
Mikulskis A, Aristarkhov A, Lin ECC (1997) Regulation of expression of ethanol dehydrogenase gene (adhE) in Escherichia coli by catabolite repressor activator protein Cra. J Bacteriol 179:7129–7134
Moat GA, Foster JW, Spector MP (2002) In: Microbial physiology, 4th edn. Wiley, New York
Nègre D, Bonod-Bidaud C, Geourjon C, Deleage G, Cozzone AJ, Cortay JC (1996) Definition of a consensus DNA-binding site for the Escherichia coli pleiotropic regulatory protein, FruR. Mol Microbiol 21(2):257–266
Park S-J, McCabe J, Turna J, Gunsalus RP (1994) Regulation of the cytrate synthase (gltA) gene of Escherichia coli in response to anaerobiosis and carbon supply: role of the arcA gene product. J Bacteriol 176:5086–5092
Peng L, Shimizu K (2003) Global metabolic regulation of Escherichia coli based on protein expression by 2-dimensional electrophoresis and enzyme activity measurement. Appl Microbiol Biotechnol 61:163–178
Peng L, Shimizu K (2006) Effect of fadR gene knockout on the metabolism of Escherichia coli based on analyses of protein expressions, enzyme activities and intracellular metabolite concentrations. Enzyme Microbiol Technol 38:512–520
Perrenoud A, Sauer U (2005) Impact of global transcriptional regulation by arcA, arcB, cra, crp, cya, fnr, and mlc on glucose catabolism in E. coli. J Bacteriol 187:3171–3179
Phue JN, Noronha SB, Hottacharyya R, Wolfe AJ, Shiloach J (2005) Biotechnol Bioeng 90:805–820
Prost J-F, Negre D, Oudot C, Murakami K, Ishihama A, Cozzone AJ, Cortay J-C (1999) J Bacteriol 181:893–898
Rahman M, Shimizu K (2008) Altered acetate metabolism and biomass production in several Escherichia coli mutants lacking rpoS-dependent metabolic pathway genes. Mol Biosyst. doi:10.1039/b712023k
Rahman M, Hasan MR, Oba T, Shimizu K (2006) Effect of rpoS gene knockout on the metabolism of E. coli during exponential growth phase and early stationary phase based on gene expressions, enzyme activities and intracellular metabolite concentrations. Biotechnol Bioeng 94:585–595
Ramseier TM, Nègre D, Cortay JC, Scarabel M, Cozzone AJ, Saier MH Jr (1993) In vitro binding of the pleiotropic transcriptional regulatory protein FruR, to the fru, pps, ace, pts and icd operons of Escherichia coli and Salmonella typhimurium. J Mol Biol 234:28–44
Ramseier TM, Bledig S, Michotey V, Fegahali R, Saier MH Jr (1995) The global regulatory protein FruR modulates the direction of carbon flow in Escherichia coli. Mol Microbiol 16:1157–1169
Ramseier TM, Chien SY, Saier MH Jr (1996) Cooperative interaction between Cra and Fnr in the regulation of the cydAB operon of Escherichia coli. Curr Microbiol 33:270–274
Ryu S, Ramseier TM, Michotoy V, Saier MH Jr, Garges S (1995) Effect of the FruR regulator on transcription of the pts operon in E. coli. J Biol Chem 270:2489–2496
Saier HM Jr, Ramseier T (1996) The catabolite repressor/activator (Cra) protein of enteric bacteria. J Bacteriol 178:3411–3417
Saier HM Jr, Ramseier T, Reizer J (1997) Regulation of carbon utilization. In: Neidhardt FC (ed) Escherichia coli and Salmonella typhimurium: cellular and molecular biology. ASM, Washington, DC
Sambrook J, Russel DW (2001) Molecular cloning: a laboratory manual, 3rd edn edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Siddiquee KAZ, Araúzo-Bravo MJ, Shimizu K (2004a) Metabolic flux analysis of pykF gene knockout Escherichia coli based on 13C-labeling experiments together with measurements of enzyme activities and intracellular metabolites concentration. Appl Microbiol Biotechnol 63:407–417
Siddiquee KAZ, Araúzo-Bravo MJ, Shimizu K (2004b) Effect of pyruvate kinase (pykF-gene) knockout mutation on the control of gene expression and metabolic fluxes in Escherichia coli. FEMS Microbiol Lett 235:25–33
Tyson K, Busby S, Cole J (1997) Catabolite regulation of two Escherichia coli operons encoding nitrite reductases: role of the Cra protein. Arch Microbiol 68(3):240–244
Acknowledgments
Marcos J. Araúzo-Bravo would like to acknowledge Japanese Society for Promotion of Science (JSPS) for supporting him for this research. Dayanidhi Sarkar would like to acknowledge Japanese Government Scholarship, Monbukagakusho (Ministry of Education, Culture, Sports, Science and Technology, Japan) for supporting his research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Erko Stackebrandt.
Dayanidhi Sarkar and Khandaker Al Zaid Siddiquee have contributed equally.
Appendices
Appendix A: Gene expressions of cra gene knockout mutant
Other than the main metabolic pathway genes, several genes related to biosynthesis and transport genes were differentially regulated.
The fru operon of enteric bacteria is known to be regulated at the transcriptional level by Cra. In the absence of cra gene, the fructose operon genes such as fruK and fruB were up-regulated in the mutant. Other carbohydrate related genes such as fucose operon genes were also up-regulated in the cra mutant.
Table 3(b) shows that some of the transporter genes such as ptsH, mglC, and xylE were up-regulated in the mutant. Those are involved in the transport of glucose, galactose, and xylose. Other transporters for the amino acids such as gltL and proV which encode glutamate/aspartate and glycine betaine/l-proline transport proteins were also up regulated in the cra mutant.
Table 3(c) shows that the expression level of fabA gene which encodes the enzyme that catalyzes a key reaction from unsaturated to saturated fatty acid was up-regulated in the mutant. The fabH (β-ketoacyl-ACP synthase I) involved in the initial steps of fatty acid elongation was also up-regulated.
Table 3(d) shows that most of the genes involved in amino acids metabolism were down regulated, where most of these genes are related to the non-aromatic amino acids biosynthetic pathway. Although some aromatic amino acids synthetic genes such as trpE, and trpC were up-regulated in the mutant, the aroL, aroG, and aroC were down regulated in the mutant. The histidine biosynthesis related genes such as hisI and hisC were also down-regulated in the mutant.
Table 3(e) shows that the lysR gene which encodes LysR protein that participates in the control of several genes involved in lysine biosynthesis was up-regulated. The repressor purR of the pur operon involved in the purine biosynthesis was down regulated. This is consistent with the up-regulation of purine biosynthesis gene. The iclR gene was up-regulated, which is in accordance with the down-regulation of the glyoxylate pathway genes in the mutant. The stress related regulatory genes such as rpoS was up-regulated in the mutant. The fis gene which serves as an early signal of nutritional up-shift was also up-regulated in the mutant as compared to its parent strain. Some of the global regulatory genes such as arcA and fnr did not change in their expression patterns in the mutant as compared to the parent strain (1.02 and 0.92, which correspond to 0.0286 and −0.120 in the log2 representation, respectively, data not shown in Table 3).
From DNA microarray data for cra gene knockout mutant, the Cra protein might be responsible for the repression of not only the fru and mlt operons but also other operons such as fuc and xyl. The up-regulation of fru operon in the mutant is consistent with the previous study, since this operon is under the negative control of Cra protein. Ramseier et al. (1995) showed that carbohydrate, oligosaccharides, monosaccharides, carbohydrate acids, and carbophosphate ester are utilized via glycolysis and by the PP pathway under the negative control of Cra protein. Fucose and xylose are usually utilized via fuc and xyl operon, and later ultimately metabolized via glycolysis and PP pathway. In the absence of Cra protein, these two operons are highly expressed in the mutant [Table 3(a)]. It was also observed that most of the carbohydrate transport genes such as mglC and xylE that are related to the metabolism through glycolysis and PP pathway were up-regulated [Table 3(b)]. Another important change was observed in the fatty acid biosynthesis, where the genes of fab operon were up-regulated. Usually fab operon is under the positive control of iclR gene, which represses the aceBAK operon for the control of the carbon flow toward the fatty acid biosynthesis. The up-regulation of iclR gene and the down-regulation of aceBAK operon genes in the mutant are consistent with the up-regulation of fatty acid biosynthesis genes.
Appendix B
Method for finding the Cra-binding sites
The method of Berg and von Hippel (1987) was implemented in Matlab® to score the E. coli genome sequence with the Cra weight matrix developed from foot-printed promoter regions of fourteen Cra-controlled operons. As in Liu and Wulf (2004) for ArcA-P, this method was used for screening the E. coli genome in order to predict the affinity of Cra for any 16-bp DNA motif sequence based on the Cra motif statistics of the input promoter regions. By determining the base frequency of the foot-printed sequences of the promoter regions, these sequences were converted into a weight matrix. In order to obtain the statistical properties of the Cra-binding site, both strands of the E. coli K-12 MG1655 genome sequence, obtained from EcoCyc™ (Keseler et al. 2005) were screened. The Cra weight matrix was calibrated by measuring the mean μ i and the standard deviation σ i for the set of foot-printed input sites. Two standard deviations σ i below the mean μ i were used as cut off for the search of potential Cra-binding sites. The potential promoter region for searching the 16-bp motif was chosen in the window of 500-bp upstream to 200-bp downstream of the start codon of each analyzed gene.
In the past research (Ramseier et al. 1993, 1995), a recognition consensus sequence has been already identified based on the foot-printed binding sites of 11 operons fru O 1 , fru O 2 , ptsH, edd-eda, gapB (epd), mtlA, pykF, ppsA, icd, aceB, and pckA (Table 4). Such consensus sequence is an incomplete asymmetric palindrome, in which the left half-site of the palindrome is conserved considerably better than the right half site. In order to refine the density distribution function associated with Cra weight matrix, we used three additional foot-printed binding sites of adhE (Mikulskis et al. 1997), pfkA (Nègre et al. 1996) and nirB (Tyson et al. 1997) operons (Table 4) and obtained a new Cra weight matrix.
Detection of the Cra-binding site
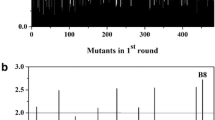
In order to find the Cra-binding region, each of the 4,639,675 successive 16-bp stretches of the E. coli K-12 MG1655 genome obtained from EcoCyc™ (Keseler et al. 2005) was scored on both strands for its degree of matching with the Cra weight matrix. The log transformation of Berg and von Hippel (1987) was used to obtain the scores distribution of all potential Cra recognition sites. From this distribution, the genomic mean (μ g = −26.4) and the standard deviation (σ g = 4.81) were obtained in order to calculate the Z transformation. A histogram window comprising the best Z scored sites (Z ≥ 3.8) is shown in Fig. 6. The Z value for the mean score μ i of the Cra recognition sites of the 14 input foot printed binding sites is also given in Fig. 6.
Z score based distribution of matrix-identified Cra recognition sites above Z ≥ 3.8. μ i and σ i denote, respectively, the mean and the standard deviation of the Cra-binding sites presented in the promoter regions of the 14 Cra-controlled input operons (Table 2). The positions of μ i ± kσ i (k = 0, 1, 2) have been Z-transformed to be compared with the genomic Z score
A total of 83 binding sites with scores above the cut-off threshold μ i − 2σ i were detected, which shows several modes of regulations, i.e., there are such genes as glk, mdtA, and b4447 which have multiple binding sites, indicating the possibility to be expressed or repressed by Cra. There are also such binding sites as located between two oppositely transcribed operons (we denote them by ↔), i.e., aroP ↔ pdhR, adhE ↔ ychE, b1425 ↔ rimL, marRAB ↔ marC, etc., which means that the unique Cra-binding site allows regulating the expressions of two operons, simultaneously. Taking into account these features, 97 different genes are potentially targeted by Cra. Using the experimental evidence, the sites can be classified into three categories. The first one contains the 14 foot-printed binding sites (corresponding to 13 genes) of Table 4, whose scores are shown in Fig. 6. The remaining 69 binding sites corresponding to 84 genes are newly found, and classified according to the degree of confidence of the microarray analysis. The second category contains 41 binding sites of the 51 genes (glk with two binding sites) as given in Table 5, whose p value of the microarray analysis data passed the Student’s t test. From this category, Fig. 7 shows the putative structure of the regulatory transcriptional unit of the genes with more salient implications in the amino acid (mhpDEF, gshA, menFD), carbon and energy metabolism (idnDOTR, adk, cyoABCDE), metabolic transport (aroP, manXYZ) and metabolic regulation (marRAB, ygfI). The last category comprises the remaining 28 new binding sites (data not shown) whose corresponding 33 genes of microarray analyses could not pass the Student’s t test.
The gene regulatory patterns. The ORFs of genes (operons) are represented as box-arrows. The region from 500 bp upstream to 200 bp downstream of each ORF is represented by a straight line with tick bars spaced at 100-bp intervals. The black arrows identify the position of documented transcriptional start sites. The small black squares represent the theoretical −10, −35 transcription region. The open circles identify the position of predicted Cra-binding sites. The operon organizations and the position of the transcription start sites were obtained from EcoCyc™. The putative binding sites were identified using our own implementation on Matlab® of the method of Berg and von Hippel
Rights and permissions
About this article
Cite this article
Sarkar, D., Siddiquee, K.A.Z., Araúzo-Bravo, M.J. et al. Effect of cra gene knockout together with edd and iclR genes knockout on the metabolism in Escherichia coli . Arch Microbiol 190, 559–571 (2008). https://doi.org/10.1007/s00203-008-0406-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-008-0406-2