Abstract
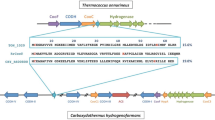
Magnesium chelatase is the first unique enzyme of the bacteriochlorophyll biosynthetic pathway. It consists of three subunits (BchI, BchD, and BchH). Amino acid sequence analysis of the Rhodobacter capsulatus BchH revealed a novel cysteine motif (393CX2CX3CX14C) that was found in only six other proteobacteria (CX2CX3CX11–14C). The cysteine motif is likely to coordinate an unprecedented [Fe–S] cluster. Purified BchH demonstrated absorbance in the 460 nm region. This absorbance was abolished in BchH proteins with alanine substitutions at positions Cys396 and Cys414. These modified proteins were also EPR silent. In contrast, wild type BchH protein in the reduced state showed EPR signals resembling those of a [4Fe–4S] cluster with rhombic symmetry and g values at 1.90, 1.93, and 2.09, superimposed with a [3Fe–4S] cluster centered at g = 2.02. The [3Fe–4S] signal was observed independently of the [4Fe–4S] signal under oxidizing conditions. Mg-chelatase activity assays showed that the cluster is not catalytic. We suggest that the [4Fe–4S] and [3Fe–4S] signals originate from a single coordination site on the monomeric BchH protein and that the [4Fe–4S] cluster is sensitive to oxidation. It is speculated that the cluster participates in the switching between aerobic and anaerobic life of the proteobacteria.







Similar content being viewed by others
Abbreviations
- AAA:
-
ATPase associated with various cellular activities
- ALA:
-
Aminolevulinate
- DTT:
-
Dithiothreitol
- IPTG:
-
Isopropyl β-d-1-thiogalactopyranoside
- MgPME:
-
Magnesium protoporphyrin monomethylester
- PMS:
-
Phenazine methosulfate
- PMSF:
-
Phenylmethylsulfonyl fluoride
- PnSB:
-
Purple nonsulfur bacteria
References
Agarwalla S, Stroud RM, Gaffney BJ (2004) Redox reactions of the iron-sulfur cluster in a ribosomal RNA methyltransferase, RumA: optical and EPR studies. J Biol Chem 279:34123–34129
Axelsson E, Lundqvist J, Sawicki A, Nilsson S, Schröder I, Al-Karadaghi S, Willows RD, Hansson M (2006) Recessiveness and dominance in barley mutants deficient in Mg-chelatase subunit D, an AAA protein involved in chlorophyll biosynthesis. Plant Cell 18:3606–3616
Bauer CE, Bird TH (1996) Regulatory circuits controlling photosynthesis gene expression. Cell 85:5–8
Beinert H, Holm RH, Munck E (1997) Iron-sulfur clusters: nature’s modular, multipurpose structures. Science 277:653–659
Beinert H, Kennedy MC, Stout CD (1996) Aconitase as iron-sulfur protein, enzyme, and iron-regulatory protein. Chem Rev 96:2335–2374
Beja O, Suzuki MT, Heidelberg JF, Nelson WC, Preston CM, Hamada T, Eisen JA, Fraser CM, DeLong EF (2002) Unsuspected diversity among marine aerobic anoxygenic phototrophs. Nature 415:630–633
Biel AJ (1992) Oxygen-regulated steps in the Rhodobacter capsulatus tetrapyrrole biosynthetic pathway. J Bacteriol 174:5272–5274
Biel AJ, Marrs BM (1983) Transcriptional regulation of several genes for bacteriochlorophyll biosynthesis in Rhodopseudomonas capsulatus in response to oxygen. J Bacteriol 156:686–694
Bingemann R, Klein A (2000) Conversion of the central [4Fe-4S] cluster into a [3Fe-4S] cluster leads to reduced hydrogen-uptake activity of the F420-reducing hydrogenase of Methanococcus voltae. Eur J Biochem 267:6612–6618
Bollivar DW, Suzuki JY, Beatty JT, Dobrowolski JM, Bauer CE (1994) Directed mutational analysis of bacteriochlorophyll a biosynthesis in Rhodobacter capsulatus. J Mol Biol 237:622–640
Brown NM, Kennedy MC, Antholine WE, Eisenstein RS, Walden WE (2002) Detection of a [3Fe-4S] cluster intermediate of cytosolic aconitase in yeast expressing iron regulatory protein 1. Insights into the mechanism of Fe-S cluster cycling. J Biol Chem 277:7246–7254
Cammack R (1992) Iron-sulfur clusters in enzymes: themes and variations. Adv Inorg Chem 38:281–322
Cohen-Bazire G, Sistrom WR, Stanier RY (1957) Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol 49:25–68
Conover RC, Kowal AT, Fu WG, Park JB, Aono S, Adams MW, Johnson MK (1990) Spectroscopic characterization of the novel iron-sulfur cluster in Pyrococcus furiosus ferredoxin. J Biol Chem 265:8533–8541
Coomber SA, Chaudhri M, Connor A, Britton G, Hunter CN (1990) Localized transposon Tn5 mutagenesis of the photosynthetic gene cluster of Rhodobacter sphaeroides. Mol Microbiol 4:977–989
Cunningham RP, Asahara H, Bank JF, Scholes CP, Salerno JC, Surerus K, Munck E, McCracken J, Peisach J, Emptage MH (1989) Endonuclease III is an iron-sulfur protein. Biochemistry 28:4450–4455
Dailey HA, Finnegan MG, Johnson MK (1994) Human ferrochelatase is an iron-sulfur protein. Biochemistry 33:403–407
Dailey TA, Dailey HA (2002) Identification of [2Fe-2S] clusters in microbial ferrochelatases. J Bacteriol 184:2460–2464
Falk JE (1964) Porphyrins and metalloporphyrins. Elsevier Publishing Company, Amsterdam
Ferreira G, Franco R, Lloyd S, Pereira A, Moura I, Moura J, Huynh B (1994) Mammalian ferrochelatase, a new addition to the metalloenzyme family. J Biol Chem 269:7062–7065
Fling SP, Gregerson DS (1986) Peptide and protein molecular weight determination by electrophoresis using a high-molarity tris buffer system without urea. Anal Biochem 155:83–88
Fodje MN, Hansson A, Hansson M, Olsen JG, Gough S, Willows RD, Al-Karadaghi S (2001) Interplay between an AAA module and an integrin I domain may regulate the function of magnesium chelatase. J Mol Biol 311:111–122
Gibson LCD, Willows RD, Kannanagara CG, von Wettstein D, Hunter CN (1995) Magnesium-protoporphyrin chelatase of Rhodobacter sphaeroides: reconstitution of activity by combining the products of the bchH, -I, and -D genes expressed in Escherichia coli. Proc Natl Acad Sci USA 92:1941–1944
Gorchein A (1973) Control of magnesium protoporphyrin chelatase activity in Rhodopsedomonas spheroides. Biochem J 134:833–845
Guigliarelli B, Bertrand P (1999) Application of EPR spectroscopy to the structural and functional study of iron-sulfur proteins. Adv Inorg Chem 47:421–497
Higgins D, Thompson J, Gibson T, Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting,position-specific gap penalties and weight matrix choice. Nucl Acids Res 22:4673–4680
Hinks JA, Evans MCW, de Miguel Y, Sartori AA, Jiricny J, Pearl LH (2002) An iron-sulfur cluster in the family 4 uracil-DNA glycosylases. J Biol Chem 277:16936–16940
Hägerhäll C, Sled V, Hederstedt L, Ohnishi T (1995) The trinuclear iron-sulfur cluster S3 in Bacillus subtilis succinate:menaquinone reductase; effects of a mutation in the putative cluster ligation motif on enzyme activity and EPR properties. Biochim Biophys Acta 1129:356–362
Leech HK, Raux E, McLean KJ, Munro AW, Robinson NJ, Borrelly GPM, Malten M, Jahn D, Rigby SEJ, Heathcote P, Warren MJ (2003) Characterization of the cobaltochelatase CbiXL: evidence for a 4Fe-4S center housed within an MXCXXC motif. J Biol Chem 278:41900–41907
Liu A, Gräslund A (2000) Electron paramagnetic resonance evidence for a novel interconversion of [3Fe-4S]+ and [4Fe-4S]+ clusters with endogenous iron and sulfide in anaerobic ribonucleotide reductase activase in vitro. J Biol Chem 275:12367–12373
Madigan MT, Gest H (1978) Growth of a photosynthetic bacterium anaerobically in darkness, supported by “oxidant-dependent” sugar fermentation. Arch Microbiol 117:119–122
Madigan MT, Gest H (1979) Growth of the photosynthetic bacterium Rhodopseudomonas capsulata chemoautotrophically in darkness with H2 as the energy source. J Bacteriol 137:524–530
Mandori A, Cecchini G, Schröder I, Gunsalus RP, Werth MT, Johnson MK (1992) [3Fe-S] to [4Fe-S] cluster conversion in Escherichia coli fumarate reductase by site-directed mutagenesis. Biochemistry 31:2703–2712
Morgan TV, Stephens PJ, Devlin F, Burgess BK, Stout CD (1985) Selective oxidative destruction of iron-sulfur clusters. Ferricyanide oxidation of Azotobacter vinelandii ferredoxin I. FEBS Lett 183:206–210
Nakamaru-Ogiso E, Yano T, Ohnishi T, Yagi T (2002) Characterization of the iron-sulfur cluster coordinated by a cysteine cluster motif (CXXCXXXCX27C) in the Nqo3 subunit in the proton-translocating NADH-quinone oxidoreductase (NDH-1) of Thermus thermophilus HB-8. J Biol Chem 277:1680–1688
Neuhoff V, Arold N, Taube D, Ehrhardt W (1988) Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis 9:255–262
Ouchane S, Steunou A, Picaud M, Astier C (2004) Aerobic and anaerobic Mg-protoporphyrin monomethyl ester cyclases in purple bacteria. J Biol Chem 279:6385–6394
Pinta V, Picaud M, Reiss-Husson F, Astier C (2002) Rubrivivax gelatinosus acsF (previously orf358) codes for a conserved, putative binuclear-iron-cluster-containing protein involved in aerobic oxidative cyclization of Mg-protoporphyrin IX monomethylester. J Bacteriol 184:746–753
Raux-Deery E, Leech HK, Nakrieko K-A, McLean KJ, Munro AW, Heathcote P, Rigby SEJ, Smith AG, Warren MJ (2005) Identification and characterization of the terminal enzyme of siroheme biosynthesis from Arabidopsis thaliana: a plastid-located sirohydrochlorin ferrochelatase containing a 2Fe-2S center. J Biol Chem 280:4713–4721
Rothery RA, Weiner JH (1993) Topological characterization of Escherichia coli DMSO reductase by electron paramagnetic resonance spectroscopy of an engineered [3Fe-4S] cluster. Biochemistry 32:5855–5861
Swingley WD, Sadekar S, Mastrian SD, Matthies HJ, Hao J, Ramos H, Acharya CR, Conrad AL, Taylor HL, Dejesa LC, Shah MK, O’Huallachain M E, Lince MT, Blankenship RE, Beatty JT, Touchman JW (2007) The complete genome sequence of Roseobacter denitrificans reveals a mixotrophic rather than photosynthetic metabolism. J Bacteriol 189:683–690
Waidner LA, Kirchman DL (2005) Aerobic anoxygenic photosynthesis genes and operons in uncultured bacteria in the Delaware River. Environ Micro 7:1896–1908
Willows RD, Beale SI (1998) Heterologous expression of the Rhodobacter capsulatus BchI, -D, and -H genes that encode magnesium chelatase subunits and characterization of the reconstituted enzyme. J Biol Chem 273:34206–34213
Willows RD, Gibson LCD, Kanangara CG, Hunter CN, von Wettstein D (1996) Three separate proteins constitute the magnesium chelatase of Rhodobacter sphaeroides. Eur J Biochem 235:438–443
Willows RD, Hansson M (2003) Mechanism, structure, and regulation of magnesium chelatase. In: Kadish KM, Smith KM, Guildard R (eds) The tetrapyrrole handbook II. Academic, New York, pp 1–48
Willows RD, Lake V, Roberts TH, Beale SI (2003) Inactivation of Mg chelatase during transition from anaerobic to aerobic growth in Rhodobacter capsulatus. J Bacteriol 185:3249–3258
Yano T, Yagi T, Sled VD, Ohnishi T (1995) Expression and characterization of the 66-kilodalton (NQO3) iron-sulfur subunit of the proton-translocating NADH-quinone oxidoreductase of Paracoccus denitrificans. J Biol Chem 270:18264–18270
Acknowledgments
We thank Dr. Cecilia Hagerhäll for fruitful discussions. NS greatly acknowledges a fellowship from the Sven and Lilly Lawski Foundation. This work was supported by the Swedish Research Council (MH, FM, and SS), the Swedish National Energy Administration, DESS and the Knut and Alice Wallenberg Foundation (FM and SS).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Theo Hansen.
Rights and permissions
About this article
Cite this article
Sirijovski, N., Mamedov, F., Olsson, U. et al. Rhodobacter capsulatus magnesium chelatase subunit BchH contains an oxygen sensitive iron–sulfur cluster. Arch Microbiol 188, 599–608 (2007). https://doi.org/10.1007/s00203-007-0282-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-007-0282-1