Abstract
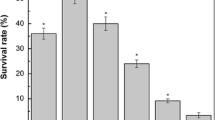
The food pathogen Bacillus cereus is likely to encounter acidic environments (i) in food when organic acids are added for preservation purposes, and (ii) during the stomachal transit of aliments. In order to characterise the acid stress response of B. cereus ATCC14579, cells were grown in chemostat at different pH values (pHo from 9.0 to 5.5) and different growth rates (μ from 0.1 to 0.8 h−1), and were submitted to acid shock at pH 4.0. Cells grown at low pHo were adapted to acid media and induced a significant acid tolerance response (ATR). The ATR induced was modulated by both pHo and μ, and the μ effect was more marked at pHo 5.5. Intracellular pH (pHi) was affected by both pHo and μ. At a pHo above 6, the pHi decreased with the decrease of pHo and the increase of μ. At pHo 5.5, pHi was higher compared to pHo 6.0, suggesting that mechanisms of pHi homeostasis were induced. The acid survival of B. cereus required protein neo-synthesis and the capacity of cells to maintain their pHi and ΔpH (pHi - pHo). Haemolysin BL and non-haemolytic enterotoxin production were both influenced by pHo and μ.







Similar content being viewed by others
Abbreviations
- ATR:
-
Acid tolerance response
- cFDASE:
-
Carboxyfluorescein diacetate succinimidyl ester
- cFSE:
-
Carboxyfluorescein succinimidyl ester
- HBL:
-
Haemolysin BL
- JB:
-
J broth
- NHE:
-
Non-haemolytic enterotoxin
- pHi :
-
Internal pH
- pHo :
-
External pH
- ΔpH,:
-
Delta pH
- GLM:
-
General linear model
- HDS:
-
Honest significant difference
References
Agata N, Ohta M, Mori M, Isobe M (1995) A novel dodecadepsipeptide, cereulide, is an emetic toxin of Bacillus cereus. FEMS Microbiol Lett 129:17–20
Belli WA, Marquis RE (1991) Adaptation of Streptococcus mutans and Enterococcus hirae to acid stress in continuous culture. Appl Environ Microbiol 57:1134–1138
Breeuwer P, Drocourt J, Rombouts F, Abee T (1996) A novel method for continuous determination of the intracellular pH in bacteria with the internally conjugated fluorescent probe 5 (and 6-)-carboxyfluorescein succinimidyl ester. Appl Environ Microbiol 62:178–183
Browne N, Dowds BC (2002) Acid stress in the food pathogen Bacillus cereus. J Appl Microbiol 92:404–414
Carlin F, Guinbretière MH, Choma CD, Pasqualini R, Braconnier A, Nguyen-The C (2000) Spore-forming bacteria in commercial cooked, pasteurized and chilled vegetable purées. Food Microbiol 17:153–165
Choma C et al (2000) Prevalence, characterization and growth of Bacillus cereus in commercial cooked chilled foods containing vegetables. J Appl Microbiol 88:617–625
Claus D, Berkeley RCW (1986) Endospore-forming gram-positive rods and cocci. In: Sneath PHA, Mair NS, Sharpe ME, Holt JG (eds) Bergey’s manual of systematic bacteriology. Williams & Wilkins, Baltimore, pp 1104–1139
Clavel T, Carlin F, Lairon D, Nguyen-The C, Schmitt P (2004) Survival of Bacillus cereus spores and vegetative cells in acid media simulating human stomach. J Appl Microbiol 97:214–219
Cotter PD, Hill C (2003) Surviving the acid test: responses of gram-positive bacteria to low pH. Microbiol Mol Biol Rev 67:429–453
Datta AR, Benjamin MM (1997) Factors controlling acid tolerance of Listeria monocytogenes: effects of nisin and other ionophores. Appl Environ Microbiol 63:4123–4126
Davis MJ, Coote PJ, O’Byrne CP (1996) Acid tolerance in Listeria monocytogenes: the adaptive acid tolerance response (ATR) and growth-phase-dependent acid resistance. Microbiology 142:2975–2982
Duport C, Thomassin S, Bourel G, Schmitt P (2004) Anaerobiosis and low specific growth rates enhance hemolysin BL production by Bacillus cereus F4430/73. Arch Microbiol 182:90–95
Jobin MP, Clavel T, Carlin F, Schmitt P (2002) Acid tolerance response is low-pH and late-stationary growth phase inducible in Bacillus cereus TZ415. Int J Food Microbiol 79:65–73
Karem KL, Foster JW, Bej AK (1994) Adaptive acid tolerance response (ATR) in Aeromonas hydrophila. Microbiology 140:1731–1736
Kramer JM, Gilbert RJ (1989) Bacillus cereus and other Bacillus species. In: Doyle MP (ed) Foofborn bacterial pathogens. Dekker M, New York, pp 21–70
Lund T, Granum PE (1997) Comparison of biological effect of the two different enterotoxin complexes isolated from three different strains of Bacillus cereus. Microbiology 143:3329–3336
Lund T, De Buyser ML, Granum PE (2000) A new cytotoxin from Bacillus cereus that may cause necrotic enteritis. Mol Microbiol 38:254–261
Minton KW, Karmin P, Hahn GM, Minton AP (1982) Nonspecific stabilization of stress-susceptible proteins by stress-resistant proteins: a model for the biological role of heat shock proteins. Proc Natl Acad Sci USA 79:7107–7111
Nascimento MM, Lemos JAC, Abranches J, Goncalves RB, Burne RA (2004) Adaptive acid tolerance response of Streptococcus sobrinus. J Bacteriol 186:6383–6390
O’Driscoll B, Gahan CG, Hill C (1996) Adaptive acid tolerance response in Listeria monocytogenes: isolation of an acid-tolerant mutant which demonstrates increased virulence. Appl Environ Microbiol 62:1693–1698
O’Hara GW, Glenn AR (1994) The adaptive acid tolerance response in root nodule bacteria and Escherichia coli. Arch Microbiol 161:286–292
O’Sullivan E, Condon S (1999) Relationship between acid tolerance, cytoplasmic pH, and ATP and H + -ATPase levels in chemostat cultures of Lactococcus lactis. Appl Environ Microbiol 65:2287–2293
Rao M, Streur TL, Aldwell FE, Cook GM (2001) Intracellular pH regulation by Mycobacterium smegmatis and Mycobacterium bovis BCG. Microbiology 147:1017–1024
Roberts D, Watson GN, Gilbert RJ (1982) Contamination of food plants and plant products with bacteria of public health significance. In: Rhodes-Roberts ME, Skinner FA (eds) Bacteria and Plants. Academic, London, pp 169–195
Siegumfeldt H, Rechinger KB, Jakobsen M (1999) Use of fluorescence ratio imaging for intracellular pH determination of individual bacterial cells in mixed cultures. Microbiology 145:1703–1709
Siegumfeldt H, Rechinger KB, Jakobsen M (2000) Dynamic changes of intracellular pH in individual lactic acid bacterium cells in response to a rapid drop in extracellular pH. Appl Environ Microbiol 66:2330–2335
Sutherland AD, Limond AM (1993) Influence of pH and sugars on the growth and production of diarrhoeagenic toxin by Bacillus cereus. J Dairy Res 60:575–580
Tiwari RP, Sachdeva N, Hoondal GS, Grewal JS (2004) Adaptive acid tolerance response in Salmonella enterica serovar Typhimurium and Salmonella enterica serovar Typhi. J Basic Microbiol 44:137–146
Ultee A, Kets EPW, Smid EJ (1999) Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl Environ Microbiol 65:4606–4610
Valero M, Fernandez PS, Salmeron MC (2003) Influence of pH and temperature on growth of Bacillus cereus in vegetable substrates. Int J Food Microbiol 82:71–79
Acknowledgements
We are grateful to Claire Dargaignaratz for her technical assistance. This work was supported by the Ministère de l’Education Nationale, de l’Enseignement Supérieur et de la Recherche (French Ministry for Education and Research).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thomassin, S., Jobin, M.P. & Schmitt, P. The acid tolerance response of Bacillus cereus ATCC14579 is dependent on culture pH, growth rate and intracellular pH. Arch Microbiol 186, 229–239 (2006). https://doi.org/10.1007/s00203-006-0137-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-006-0137-1