Abstract
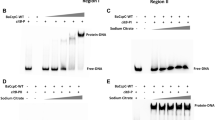
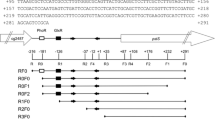
The tricarboxylic acid (TCA) cycle is one of the major routes of carbon catabolism in Bacillus subtilis. The syntheses of the enzymes performing the initial reactions of the cycle, citrate synthase, and aconitase, are synergistically repressed by rapidly metabolizable carbon sources and glutamine. This regulation involves the general transcription factor CcpA and the specific repressor CcpC. In this study, we analyzed the expression and intracellular localization of CcpC. The synthesis of citrate, the effector of CcpC, requires acetyl-CoA. This metabolite is located at a branching point in metabolism. It can be converted to acetate in overflow metabolism or to citrate. Manipulations of the fate of acetyl-CoA revealed that efficient citrate synthesis is required for the expression of the citB gene encoding aconitase and that control of the two pathways utilizing acetyl-CoA converges in the control of citrate synthesis for the induction of the TCA cycle. The citrate pool seems also to be controlled by arginine catabolism. The presence of arginine results in a severe CcpC-dependent repression of citB. In addition to regulators involved in sensing the carbon status of the cell, the pleiotropic nitrogen-related transcription factor, TnrA, activates citB transcription in the absence of glutamine.


Similar content being viewed by others
Abbreviations
- TCA:
-
Tricarboxylic acid
References
Alén C, Sonenshein AL (1999) Bacillus subtilis aconitase is an RNA-binding protein. Proc Natl Acad Sci USA 96:10412–10417
Atkinson MR, Fisher SH (1991) Identification of genes and gene products whose expression is activated during nitrogen-limited growth in Bacillus subtilis. J Bacteriol 173:23–27
Belitsky BR (2002) Biosynthesis of amino acids of the glutamate and aspartate families, alanine and polyamines. In: Sonenshein AL, Hoch JA, Losick R (eds) Bacillus subtilis and its closest relatives: from genes to cells. American Society for Microbiology Press, Washington, DC pp 203–231
Belitsky BR, Sonenshein AL (1998) Role and regulation of Bacillus subtilis glutamate dehydrogenase genes. J Bacteriol 180:6298–6305
Belitsky BR, Wray LV, Fisher SH, Bohannon DE, Sonenshein AL (2000) Role of TnrA in nitrogen source-dependent repression of Bacillus subtilis glutamate synthase gene expression. J Bacteriol 182:5939–5947
Belitsky BR, Sonenshein AL (2004) Modulation of activity of Bacillus subtilis regulatory proteins GltC and TnrA by glutamate dehydrogenase. J Bacteriol 186:3399–3407
Blencke HM, Homuth G, Ludwig H, Mäder U, Hecker M, Stülke J (2003) Transcriptional profiling of gene expression in response to glucose in Bacillus subtilis: regulation of the central metabolic pathways. Metab Engn 5:133–149
Bohannon DE, Sonenshein AL (1989) Positive regulation of glutamate biosynthesis in Bacillus subtilis. J Bacteriol 171:4718–4727
Böhm A, Boos W (2004) Transport-dependent gene regulation by sequestration of transcriptional regulators. Top Curr Genet 9:47–66
Coutts G, Thomas G, Blakey D, Merrick M (2002) Membrane sequestration of the signal transduction protein GlnK by the ammonium transporter AmtB. EMBO J 21:536–545
Dauner M, Storni T, Sauer U (2001) Bacillus subtilis metabolism and energetics in carbon-limited and excess-carbon chemostat culture. J Bacteriol 183:7308–7317
Dean AM, Lee MH, Koshland DE Jr (1989) Phosphorylation inactivates Escherichia coli isocitrate dehydrogenase by preventing isocitrate binding. J Biol Chem 264:20482–20486
Detsch C, Stülke J (2003) Ammonium utilization in Bacillus subtilis: transport and regulatory functions of NrgA and NrgB. Microbiology 149:3289–3297
Deutscher J, Galinier A, Martin-Verstraete I (2002) Carbohydrate uptake and metabolism. In: Sonenshein AL, Hoch JA, Losick R (eds) Bacillus subtilis and its closest relatives: from genes to cells. American Society for Microbiology Press, Washington, DC, pp 129–150
Eymann C, Dreisbach A, Albrecht D, Bernhardt J, Becher D, Gentner S, Le Thi T, Büttner K, Buurmann G, Scharf C, Venz S, Völker U, Hecker M (2004) A comprehensive proteome map of growing Bacillus subtilis cells. Proteomics 4:2849–2876
Faires N, Tobisch S, Bachem S, Martin-Verstraete I, Hecker M, Stülke J (1999) The catabolite control protein CcpA controls ammonium assimilation in Bacillus subtilis. J Mol Microbiol Biotechnol 1:141–148
Fillinger S, Boschi-Muller S, Azza S, Dervyn E, Branlant G, Aymerich S (2000) Two glyceraldehyde−3-phosphate dehydrogenases with opposite physiological roles in a nonphotosynthetic bacterium. J Biol Chem 275:14031–14037
Fisher SH, Débarbouillé M (2002) Nitrogen source utilization and its regulation. In: Sonenshein AL, Hoch JA, Losick R (eds) Bacillus subtilis and its closest relatives: from genes to cells. American Society for Microbiology Press, Washington, DC, pp 181–191
Fisher SH, Magasanik B (1984) 2-Ketoglutarate and the regulation of aconitase and histidase formation Bacillus subtilis. J Bacteriol 158:379–382
Fisher SH, Sonenshein AL (1984) Bacillus subtilis glutamine synthetase mutants pleiotropically altered in catabolite repression. J Bacteriol 157:612–621
Grundy FJ, Waters DA, Allen SH, Henkin TM (1993) Regulation of the Bacillus subtilis acetate kinase gene by CcpA. J Bacteriol 175:7348–7355
Guérout-Fleury AM, Shazand K, Frandsen N, Stragier P (1995) Antibiotic-resistance cassettes for Bacillus subtilis. Gene 167:335–336
Jourlin-Castelli C, Mani N, Nakano MM, Sonenshein AL (2000) CcpC, a novel regulator of the LysR family required for glucose repression of the citB gene in Bacillus subtilis. J Mol Biol 295:865–878
Kim HJ, Jourlin-Castelli C, Kim SI, Sonenshein AL (2002a) Regulation of the Bacillus subtilis ccpC gene by CcpA and CcpC. Mol Microbiol 43:399–410
Kim HJ, Roux A, Sonenshein AL (2002b) Direct and indirect roles of CcpA in regulation of Bacillus subtilis Krebs cycle genes. Mol Microbiol 45:179–190
Kim SI, Jourlin-Castelli C, Wellington SR, Sonenshein AL (2003) Mechanism of repression by Bacillus subtilis CcpC, a LysR family regulator. J Mol Biol 334:609–624
Krüger S, Stülke J, Hecker M (1993) Catabolite repression of β-glucanase synthesis in Bacillus subtilis. J Gen Microbiol 139:2047–2054
Ludwig H, Homuth G, Schmalisch M, Dyka FM, Hecker M. Stülke J (2001) Transcription of glycolytic genes and operons in Bacillus subtilis: evidence for the presence of multiple levels of control of the gapA operon. Mol Microbiol 41:409–422
Ludwig H, Rebhan N, Blencke HM, Merzbacher M, Stülke J (2002) Control of the glycolytic gapA operon by the catabolite control protein A in Bacillus subtilis: a novel mechanism of CcpA-mediated regulation. Mol Microbiol 45:543–553
Martin-Verstraete I, Débarbouillé M, Klier A, Rapoport G (1992) Mutagenesis of the Bacillus subtilis ‘-12,-24’ promoter of the levanase operon and evidence for the existence of an upstream activating sequence. J Mol Biol 226:85–99
Martin-Verstraete I, Débarbouillé M, Klier A, Rapoport G (1994) Interactions of wild type and truncated LevR of Bacillus subtilis with the upstream activating sequence of the levanase operon. J Mol Biol 241:178–192
Matsuno K, Blais T, Serio AW, Conway T, Henkin TM, Sonenshein AL (1999) Metabolic imbalance and sporulation in an isocitrate dehydrogenase mutant of Bacillus subtilis. J Bacteriol 181:3382–3391
Meinken C, Blencke HM, Ludwig H, Stülke J (2003) Expression of the glycolytic gapA operon in Bacillus subtilis: differential syntheses of proteins encoded by the operon. Microbiology 149:751–761
Monedero V, Poncet S, Mijakovic I, Fieulaine S, Dossonet V, Martin-Verstraete I, Nessler S, Deutscher J (2001) Mutations lowering the phosphatase activity of HPr kinase/phosphatase switch off carbon metabolism. EMBO J 20:3928–3937
Moreno M S, Schneider BL, Maile RR, Weyler W, Saier MH Jr (2001) Catabolite repression mediated by CcpA protein in Bacillus subtilis: novel modes of regulation revealed by whole-genome analyses. Mol Microbiol 39:1366–1381
Presecan-Siedel E, Galinier A, Longin R, Deutscher J, Danchin A, Glaser P, Martin-Verstraete I (1999) The catabolite regulation of the pta gene as part of carbon flow pathways in Bacillus subtilis. J Bacteriol 181:6889–6897
Rosenkrantz MS, Dingman DW, Sonenshein AL (1985) Bacillus subtilis citB gene is regulated synergistically by glucose and glutamine. J Bacteriol 164:155–164
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Saxild HH, Nygaard P (1987) Genetic and physiological characterization of Bacillus subtilis mutants resistant to purine analogs. J Bacteriol 169:2977–2983
Schirmer F, Ehrt S, Hillen W (1997) Expression, inducer spectrum, domain structure, and function of MopR, the regulator of phenol degradation in Acinetobacter calcoaceticus NCIB8250. J Bacteriol 179:1329–1336
Singh SK, Miller SP, Dean A, Banaszak LJ, LaPorte DC (2002) Bacillus subtilis isocitrate dehydrogenase. A substrate analogue for Escherichia coli isocitrate dehydrogenase kinase/phosphatase. J Biol Chem 277:7567–7573
Sonenshein AL (2002) The Krebs citric acid cycle. In: Sonenshein AL, Hoch JA, Losick R (eds) Bacillus subtilis and its closest relatives: from genes to cells. American Society for Microbiology Press, Washington, DC, pp 151–162
Stülke J, Hillen W (2000) Regulation of carbon catabolism in Bacillus species. Annu Rev Microbiol 54:849–880
Tobisch S, Zühlke D, Bernhardt J, Stülke J, M Hecker (1999) Role of CcpA in regulation of the central pathways of carbon catabolism in Bacillus subtilis. J Bacteriol 181:6996–7004
Wacker I, Ludwig H, Reif I, Blencke HM, Detsch C, Stülke J (2003) The regulatory link between carbon and nitrogen metabolism in Bacillus subtilis: regulation of the gltAB operon by the catabolite control protein CcpA. Microbiology 149:3001–3009
Weinrauch Y, Msadek T, Kunst F, Dubnau D (1991) Sequence and properties of comQ, a new competence regulatory gene of Bacillus subtilis. J Bacteriol 173:5685–5693
Wray LV Jr, Ferson AE, Rohrer K, Fisher SH (1996) TnrA, a transcription factor required for global nitrogen regulation in Bacillus subtilis. Proc Natl Acad Sci USA 93:8841–8845
Wray LV Jr, Zalieckas JM, Fisher SH (2001) Bacillus subtilis glutamine synthetase controls gene expression through protein-protein interaction with transcription factor TnrA. Cell 107:427–435
Yoshida KI, Kobayashi K, Miwa Y, Kang CM, Matsunaga M, Yamaguchi Y, Tojo S, Yamamoto M, Nishi R, Ogasawara N, Nakayama T, Fujita Y (2001) Combined transcriptome and proteome analysis as a powerful approach to study genes under glucose repression in Bacillus subtilis. Nucl Acids Res 29:6683–6692
Acknowledgments
We are grateful to Sabine Lentes for her help with some of the experiments. Linc Sonenshein is acknowledged for the critical reading of the manuscript. This work was supported by grants from the Deutsche Forschungsgemeinschaft (Stu 214/2-1; Stu 214/2-2) and the Fonds der Chemischen Industrie.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blencke, HM., Reif, I., Commichau, F.M. et al. Regulation of citB expression in Bacillus subtilis: integration of multiple metabolic signals in the citrate pool and by the general nitrogen regulatory system. Arch Microbiol 185, 136–146 (2006). https://doi.org/10.1007/s00203-005-0078-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-005-0078-0