Abstract
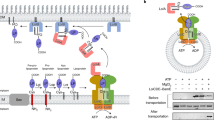
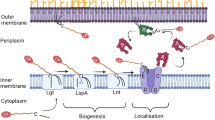
Bacterial lipoproteins comprise a subset of membrane proteins that are covalently modified with lipids at the amino-terminal Cys. Lipoproteins are involved in a wide variety of functions in bacterial envelopes. Escherichia coli has more than 90 species of lipoproteins, most of which are located on the periplasmic surface of the outer membrane, while others are located on that of the inner membrane. In order to elucidate the mechanisms by which outer-membrane-specific lipoproteins are sorted to the outer membrane, biochemical, molecular biological and crystallographic approaches have been taken. Localization of lipoproteins on the outer membrane was found to require a lipoprotein-specific sorting machinery, the Lol system, which is composed of five proteins (LolABCDE). The crystal structures of LolA and LolB, the periplasmic chaperone and outer-membrane receptor for lipoproteins, respectively, were determined. On the basis of the data, we discuss here the mechanism underlying lipoprotein transfer from the inner to the outer membrane through Lol proteins. We also discuss why inner membrane-specific lipoproteins remain on the inner membrane.



Similar content being viewed by others
References
Braun V, Wu HC (1994) Lipoproteins, structure, function, biosynthesis and model for protein export. In: Ghuysen J-M, Hakenbeck R (eds) New comprehensive biochemistry, vol 27. Bacterial cell wall. Elsevier, Amsterdam, pp 319–341
Chen J, Sharma S, Quiocho FA, Davidson AL (2001) Trapping the transition state of an ATP-binding cassette transporter: evidence for a concerted mechanism of maltose transport. Proc Natl Acad Sci USA 98:1525–1530
DeChavigny A, Heacock PN, Dowhan W (1991) Sequence and inactivation of the pss gene of Escherichia coli. Phosphatidylethanolamine may not be essential for cell viability. J Biol Chem 266:5323–5332
Doerrler WT, Ready MC, Raetz CR (2001) An Escherichia coli mutant defective in lipid export. J Biol Chem 276:11461–11464
Driessen AJ, Manting EH, van der Does C (2001) The structural basis of protein targeting and translocation in bacteria. Nat Struct Biol 8:492–498
Duong F, Eichler J, Price A, Leonard MR, Wickner W (1997) Biogenesis of the gram-negative bacterial envelope. Cell 91:567–573
Economou A (2002) Bacterial secretome: the assembly manual and operating instructions. Mol Membr Biol 19:159–169
Fukuda A, Matsuyama S, Hara T, Nakayama J, Nagasawa H, Tokuda H (2002) Aminoacylation of the N-terminal cysteine is essential for Lol-dependent release of lipoproteins from membranes but does not depend on lipoprotein sorting signals. J Biol Chem 277:43512–43518
Gennity JM, Inouye M (1991) The protein sequence responsible for lipoprotein membrane localization in Escherichia coli exhibits remarkable specificity. J Biol Chem 266:16458–16464
Hara T, Matsuyama S, Tokuda H (2003) Mechanism underlying the inner membrane retention of E. coli lipoproteins caused by Lol avoidance signals. J Biol Chem 278:40408–40414
Hayashi S, Wu HC (1990) Lipoproteins in bacteria. J Bioenerg Biomembr 22:451–471
Masuda K, Matsuyama S, Tokuda H (2002) Elucidation of the function of lipoprotein-sorting signals that determine membrane localization. Proc Natl Acad Sci USA 99:7390–7395
Matsuyama S, Tajima T, Tokuda H (1995) A novel periplasmic carrier protein involved in the sorting and transport of Escherichia coli lipoproteins destined for the outer membrane. EMBO J 14:3365–3372
Matsuyama S, Yokota N, Tokuda H (1997) A novel outer membrane lipoprotein, LolB (HemM), involved in the LolA (p20)-dependent localization of lipoproteins to the outer membrane of Escherichia coli. EMBO J 16:6947–6955
Miyamoto A, Matsuyama S, Tokuda H (2001) Mutant of LolA, a lipoprotein-specific molecular chaperone of Escherichia coli, defective transfer of lipoprotein to LolB. Biochem Biophys Res Commun 287:1125–1128
Miyamoto A, Matsuyama S, Tokuda (2002) Dominant negative mutant of a lipoprotein-specific molecular chaperone, LolA, tightly associates with LolCDE. FEBS Lett 528:193–196
Mori H, Ito K (2001) The Sec protein-translocation pathway. Trends Microbiol 9:494–500
Muller M, Koch HG, Beck K, Schafer U (2000) Protein traffic in bacteria: multiple routes from the ribosome to and across the membrane. Prog Nucleic Acid Res Mol Biol 66:107–157
Narita S, Tanaka K, Matsuyama S, Tokuda H (2002) Disruption of lolCDE encoding an ATP-binding-cassette transporter is lethal for Escherichia coli and prevents the release of lipoproteins from the inner membrane. J Bacteriol 184:1417–1422
Narita S, Kanamaru K, Matsuyama S, Tokuda H (2003) A mutation in the membrane subunit of an ABC transporter LolCDE complex causing outer membrane localization of lipoproteins against their inner membrane-specific signals. Mol Microbiol 49:167–177
Pugsley AP (1993) The complete general secretory pathway in Gram-negative bacteria. Microbiol Rev 57:50–108
Pugsley AP, d’Enfert C, Reyss I, Kornacker MG (1990) Genetics of extracellular protein secretion by Gram-negative bacteria. Annu Rev Genet 24:67–90
Robichon C, Bonhivers M, Pugsley AP (2003) An intramolecular disulphide bond reduces the efficacy of a lipoprotein plasma membrane sorting signal. Mol Microbiol 49:1145–1154
Sankaran K, Wu HC (1994) Lipid modification of bacterial prolipoproteins. Transfer of diacylglyceryl moiety from phosphatidylglycerol. J Biol Chem 269:19701–19706
Seydel A, Gounon P, Pugsley AP (1999) Testing the ‘+2 rule’ for lipoprotein sorting in the Escherichia coli cell envelope with a new genetic selection. Mol Microbiol 34:810–821
Spurlino JC, Lu G-Y, Quiocho FA (1991) The 2.3-Å resolution structure of the maltose- or maltodextrin-binding protein, a primary receptor of bacterial active transport and chemotaxis. J Biol Chem 266:5202–5219
Tajima T, Yokota N, Matsuyama S, Tokuda H (1998) Genetic analyses of the in vivo function of LolA, a periplasmic chaperone involved in the outer membrane localization of Escherichia coli lipoproteins. FEBS Lett 439:51–54
Takeda K, Miyatake H, Yokota N, Matsuyama S, Tokuda H, Miki K (2003) Crystal structures of bacterial lipoprotein localization factors, LolA and LolB. EMBO J 22:3199–3209
Tanaka K, Matsuyama S, Tokuda H (2001) Deletion of lolB encoding an outer membrane lipoprotein is lethal for Escherichia coli and causes the accumulation of lipoprotein localization intermediates in the periplasm. J Bacteriol 183:6538–6542
Terada M, Kuroda T, Matsuyama S, Tokuda H (2001) Lipoprotein sorting signals evaluated as the LolA-dependent release of lipoproteins from the inner membrane of Escherichia coli. J Biol Chem 276:47690–47694
Yakushi T, Yokota N, Matsuyama S, Tokuda H (1998) LolA-dependent release of a lipid-modified protein from the inner membrane of Escherichia coli requires nucleotide triphosphate. J Biol Chem 273:32576–32581
Yakushi T, Masuda K, Narita S, Matsuyama S, Tokuda H (2000) A new ABC transporter mediating the detachment of lipid-modified proteins from membranes. Nat Cell Biol 2:212–218
Yamaguchi K, Yu F, Inouye M (1988) A single amino acid determinant of the membrane localization of lipoproteins in E. coli. Cell 53:423–432
Acknowledgements
We wish to thank Rika Ishihara for her help in the preparation of this review. The work described here was supported by grants to H. T. from the Ministry of Education, Science, Sports and Culture of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Narita, Si., Matsuyama, Si. & Tokuda, H. Lipoprotein trafficking in Escherichia coli . Arch Microbiol 182, 1–6 (2004). https://doi.org/10.1007/s00203-004-0682-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-004-0682-4