Abstract
Summary
Patients with Duchenne muscular dystrophy (DMD) have a high fracture burden due to progressive myopathy and steroid-induced osteoporosis. This study in males with DMD showed that markers of systemic glucocorticoid exposure including shorter stature, greater bone age delay, and lower lumbar spine bone mineral density were associated with spine fragility.
Introduction
Fragility fractures are frequent in DMD. The purpose of this study was to identify clinical factors associated with prevalent vertebral fractures (VF) in boys, teens/young adults with Duchenne muscular dystrophy (DMD).
Methods
This was a cross-sectional study of males aged 4–25 years with DMD. VF were evaluated using the modified Genant semi-quantitative method on T4-L4 lateral spine radiographs. Areal bone mineral density (aBMD) was measured at the lumbar spine (LS) and used to estimate volumetric BMD (vBMD). Clinical factors were analyzed for their association with the Spinal Deformity Index (SDI, the sum of the Genant grades).
Results
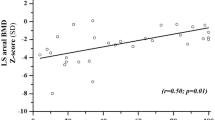
Sixty participants were enrolled (mean age 11.5 years, range 5.4–19.5). Nineteen participants (32%) had a total of 67 VF; 23/67 VF (34%) were moderate or severe. Participants with VF were shorter (mean height Z-score ± standard deviation: − 3.1 ± 1.4 vs. − 1.8 ± 1.4, p = 0.001), had longer glucocorticoid exposure (mean duration 6.0 ± 3.3 vs. 3.9 ± 3.3 years, p = 0.027), greater bone age (BA) delay (mean BA to chronological age difference − 3.2 ± 3.4 vs. − 1.3 ± 1.2 years, p = 0.035), and lower LSaBMD Z-scores (mean − 3.0 ± 1.0 vs. − 2.2 ± 1.2, p = 0.023). There was no difference in LSvBMD Z-scores.
Multivariable Poisson regression showed that every 0.1 mg/kg/day increment in average glucocorticoid daily dose was associated with a 1.4-fold SDI increase (95% confidence interval: 1.1–1.7, p = 0.013). Greater BA delay (p < 0.001), higher weight Z-score (p = 0.004), decreased height Z-score (p = 0.025), and lower LSvBMD Z-score (p = 0.025) were also associated with SDI increase.
Conclusion
Readily measurable clinical variables were associated with prevalent VF in males with glucocorticoid-treated DMD. These variables may be useful to identify candidates for primary osteoporosis prevention after glucocorticoid initiation.



Similar content being viewed by others
References
Yiu EM, Kornberg AJ (2015) Duchenne muscular dystrophy. J Paediatr Child Health 51:759–764. https://doi.org/10.1111/jpc.12868
Bello L, Morgenroth LP, Gordish-Dressman H, Hoffman EP, McDonald CM, Cirak S, investigators C, (2016) DMD genotypes and loss of ambulation in the CINRG Duchenne Natural History Study. Neurology 87:401–409. https://doi.org/10.1212/WNL.0000000000002891
McDonald CM, Henricson EK, Abresch RT et al (2018) Long-term effects of glucocorticoids on function, quality of life, and survival in patients with Duchenne muscular dystrophy: a prospective cohort study. Lancet 391:451–461. https://doi.org/10.1016/S0140-6736(17)32160-8
Joseph S, Wang C, Bushby K, Guglieri M, Horrocks I, Straub V, Ahmed SF, Wong SC (2019) Fractures and linear growth in a nationwide cohort of boys with Duchenne muscular dystrophy with and without glucocorticoid treatment. JAMA Neurol 76:701–709. https://doi.org/10.1001/jamaneurol.2019.0242
Larson CMC (2000) Bone mineral density and fractures in boys with Duchenne muscular dystrophy. J Pediatr Orthop 20:71–74
Ma J, McMillan HJ, Karagüzel G et al (2017) The time to and determinants of first fractures in boys with Duchenne muscular dystrophy. Osteoporos Int 28:597–608. https://doi.org/10.1007/s00198-016-3774-5
Cummings EA, Ma J, Fernandez CV et al (2015) Incident vertebral fractures in children with leukemia during the four years following diagnosis. J Clin Endocrinol Metab 100:3408–3417. https://doi.org/10.1210/JC.2015-2176
Ward LM, Hadjiyannakis S, McMillan HJ, Noritz G, Weber DR (2018) Bone health and psteoporosis management of the patient with Duchenne muscular dystrophy. Pediatrics 142:S34–S42. https://doi.org/10.1542/peds.2018-0333E
Birnkrant DJ, Bushby K, Bann CM et al (2018) Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol 17:347–361. https://doi.org/10.1016/S1474-4422(18)30025-5
Ward LM, Choudhury A, Alos N et al (2021) Zoledronic acid vs placebo in pediatric glucocorticoid-induced osteoporosis: a randomized, double-blind, phase 3 trial. J Clin Endocrinol Metab 106:e5222–e5235. https://doi.org/10.1210/clinem/dgab458
Zacharin M, Lim A, Gryllakis J et al (2021) Randomized controlled trial evaluating the use of zoledronic acid in Duchenne muscular dystrophy. J Clin Endocrinol Metab 106:2328–2342. https://doi.org/10.1210/clinem/dgab302
Ward LM (2020) Glucocorticoid-induced osteoporosis: why kids are different. Front Endocrinol (Lausanne) 11:576. https://doi.org/10.3389/fendo.2020.00576
Ward LM, Ma J, Robinson ME et al (2021) Osteoporotic fractures and vertebral body reshaping in children with glucocorticoid-treated rheumatic disorders. J Clin Endocrinol Metab 106:e5195–e5207. https://doi.org/10.1210/clinem/dgab494
Yildiz S, Glanzman AM, Estilow T, Flickinger J, Brandsema JF, Tennekoon G, Banwell BL, Yum S (2020) Retrospective analysis of fractures and factors causing ambulation loss after lower limb fractures in Duchenne muscular dystrophy. Am J Phys Med Rehabil 99:789–794. https://doi.org/10.1097/PHM.0000000000001423
Medeiros MO, Behrend C, King W, Sanders J, Kissel J, Ciafaloni E (2013) Fat embolism syndrome in patients with Duchenne muscular dystrophy. Neurology 80:1350–1352. https://doi.org/10.1212/WNL.0b013e31828ab313
Sbrocchi AM, Rauch F, Jacob P, McCormick A, McMillan HJ, Matzinger MA, Ward LM (2012) The use of intravenous bisphosphonate therapy to treat vertebral fractures due to osteoporosis among boys with Duchenne muscular dystrophy. Osteoporos Int 23:2703–2711. https://doi.org/10.1007/s00198-012-1911-3
Ding L, Hu J, Wang D, Liu Q, Mo Y, Tan X, Wen F (2020) Efficacy and safety of first- and second-line drugs to prevent glucocorticoid-induced fractures. J Clin Endocrinol Metab 105:1. https://doi.org/10.1210/clinem/dgz023
Amiche MA, Albaum JM, Tadrous M, Pechlivanoglou P, Lévesque LE, Adachi JD, Cadarette SM (2016) Efficacy of osteoporosis pharmacotherapies in preventing fracture among oral glucocorticoid users: a network meta-analysis. Osteoporos Int 27:1989–1998. https://doi.org/10.1007/s00198-015-3476-4
Halton J, Gaboury I, Grant R et al (2009) Advanced vertebral fracture among newly diagnosed children with acute lymphoblastic leukemia: results of the Canadian Steroid-Associated Osteoporosis in the Pediatric Population (STOPP) research program. J Bone Miner Res 24:1326–1334. https://doi.org/10.1002/jbmr.5650070204
Huber AM, Gaboury I, Cabral DA et al (2010) Prevalent vertebral fractures among children initiating glucocorticoid therapy for the treatment of rheumatic disorders. Arthritis Care Res (Hoboken) 62:516–526. https://doi.org/10.1002/acr.20171
Feber J, Gaboury I, Ni A et al (2012) Skeletal findings in children recently initiating glucocorticoids for the treatment of nephrotic syndrome. Osteoporos Int 23:751–760. https://doi.org/10.1007/s00198-011-1621-2
Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL (2002) Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics 109:45–60. https://doi.org/10.1542/peds.109.1.45
Gauld LM et al (2004) Height prediction from ulna length. Dev Med Child Neurol 46:475–480. https://doi.org/10.1017/s0012162204000787
Marshall WA, Tanner JM (1970) Variations in the pattern of pubertal changes in boys. Arch Dis Child 45:13–23. https://doi.org/10.1136/adc.45.239.13
McMillan HJ, Campbell C, Mah JK (2010) Duchenne muscular dystrophy: Canadian paediatric neuromuscular physicians survey. Can J Neurol Sci 37:195–205. https://doi.org/10.1017/s0317167100009926
Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium (2011) Dietary reference intakes for calcium and vitamin D. In: A Catharine Ross, Christine L Taylor, Ann L Yaktine, and Heather B Del Valle (eds.), Washington (DC): The National Academies Press (US). https://www.ncbi.nlm.nih.gov/books/NBK56070/. Accessed 16 March 2022
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148. https://doi.org/10.1002/jbmr.5650080915
Crabtree NJ, Shaw NJ, Bishop NJ et al (2017) Amalgamated reference data for size-adjusted bone densitometry measurements in 3598 children and young adults—the ALPHABET study. J Bone Miner Res 32:172–180. https://doi.org/10.1002/jbmr.2935
Zemel BS, Stallings VA, Leonard MB, Paulhamus DR, Kecskemethy HH, Harcke HT, Henderson RC (2009) Revised pediatric reference data for the lateral distal femur measured by Hologic Discovery/Delphi dual-energy X-ray absorptiometry. J Clin Densitom 12:207–218. https://doi.org/10.1016/j.jocd.2009.01.005
Carter DR, Bouxsein ML, Marcus R (1992) New approaches for interpreting projected bone densitometry data. J Bone Miner Res 7:137–145
Huang Y, Eapen E, Steele S, Grey V (2011) Establishment of reference intervals for bone markers in children and adolescents. Clin Biochem 44:771–778. https://doi.org/10.1016/j.clinbiochem.2011.04.008
Rauchenzauner M, Schmid A, Heinz-Erian P, Kapelari K, Falkensammer G, Griesmacher A, Finkenstedt G, Hogler W (2007) Sex- and age-specific reference curves for serum markers of bone turnover in healthy children from 2 months to 18 years. J Clin Endocrinol Metab 92:443–449. https://doi.org/10.1210/jc.2006-1706
Wang JS, Mazur CM, Wein MN (2021) Sclerostin and osteocalcin: candidate bone-produced hormones. Front Endocrinol (Lausanne) 12:584147. https://doi.org/10.3389/fendo.2021.584147
Lorenzo JA (2020) The role of interleukin-6 in bone. J Endocr Soc 4(10):bvaa112
Brooke MH, Griggs RC, Mendell JR, Fenichel GM, Shumate JB, Pellegrino RJ (1981) Clinical trial in Duchenne dystrophy. I. The design of the protocol. Muscle Nerve 4:186–197. https://doi.org/10.1002/mus.880040304
Vignos PJ Jr, Spencer GE Jr, Archibald KC (1963) Management of progressive muscular dystrophy in childhood. JAMA 184:89–96. https://doi.org/10.1001/jama.1963.03700150043007
Florence JM, Pandya S, King WM, Robison JD, Signore LC, Wentzell M, Province MA (1984) Clinical trials in Duchenne dystrophy. Standardization and reliability of evaluation procedures. Phys Ther 64:41–45. https://doi.org/10.1093/ptj/64.1.41
Varni JW, Burwinkle TM, Seid M, Skarr D (2003) The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr 3:329–341. https://doi.org/10.1367/1539-4409(2003)003%3c0329:tpaapp%3e2.0.co;2
Siminoski K, Lee K-C, Jen H, Warshawski R, Matzinger M, Shenouda N, Charron M, Coblentz C, Dubois J, Kloiber RJOI (2012) Anatomical distribution of vertebral fractures: comparison of pediatric and adult spines. Osteoporos Int 23:1999–2008. https://doi.org/10.1007/s00198-011-1837-1
McDonald CM, Henricson EK, Abresch RT et al (2013) The 6-minute walk test and other endpoints in Duchenne muscular dystrophy: longitudinal natural history observations over 48 weeks from a multicenter study. Muscle Nerve 48:343–356. https://doi.org/10.1002/mus.23902
McDonald CM (2018) Timed function tests have withstood the test of time as clinically meaningful and responsive endpoints in Duchenne muscular dystrophy. Muscle Nerve 58:614–617. https://doi.org/10.1002/mus.26334
Nakhla M, Scuccimarri R, Duffy KN, Chédeville G, Campillo S, Duffy CM, Azouz EM, Shenouda N, Sharma AK, Rodd C (2009) Prevalence of vertebral fractures in children with chronic rheumatic diseases at risk for osteopenia. J Pediatr 154:438–443. https://doi.org/10.1016/j.jpeds.2008.09.023
Tian C, Wong BL, Hornung L, Khoury JC, Miller L, Bange J, Rybalsky I, Rutter MM (2016) Bone health measures in glucocorticoid-treated ambulatory boys with Duchenne muscular dystrophy. Neuromuscul Disord 26:760–767. https://doi.org/10.1016/j.nmd.2016.08.011
Baptista CRJA, Costa AA, Pizzato TM, Souza FB, Mattiello-Sverzut AC (2014) Postural alignment in children with Duchenne muscular dystrophy and its relationship with balance. Braz J Phys Ther 18:119–126. https://doi.org/10.1590/s1413-35552012005000152
Oda T, Shimizu N, Yonenobu K, Ono K, Nabeshima T, Kyoh S (1993) Longitudinal study of spinal deformity in Duchenne muscular dystrophy. J Pediatr Orthop 13:478–488. https://doi.org/10.1097/01241398-199307000-00012
Cirillo Totera JI, Fleiderman Valenzuela JG, Garrido Arancibia JA, Pantoja Contreras ST, Beaulieu Lalanne L, Alvarez-Lemos FL (2021) Sagittal balance: from theory to clinical practice. EFORT open reviews 6:1193–1202. https://doi.org/10.1302/2058-5241.6.210062
McDonald DG, Kinali M, Gallagher AC, Mercuri E, Muntoni F, Roper H, Jardine P, Jones DH, Pike MG (2002) Fracture prevalence in Duchenne muscular dystrophy. Dev Med Child Neurol 44:695–698. https://doi.org/10.1017/s0012162201002778
Gray B, Hsu JD, Furumasu J (1992) Fractures caused by falling from a wheelchair in patients with neuromuscular disease. Dev Med Child Neurol 34:589–592. https://doi.org/10.1111/j.1469-8749.1992.tb11489.x
LeBlanc CM, Ma J, Taljaard M et al (2015) Incident vertebral fractures and risk factors in the first three years following glucocorticoid initiation among pediatric patients with rheumatic disorders. J Bone Miner Res 30:1667–1675. https://doi.org/10.1002/jbmr.2511
Funding
The Bone Fragility in Boys with DMD study was funded by the Physicians’ Services Inc. (Grant No. 14–03, awarded to LMW, Study Principal Investigator). The Steroid-associated Osteoporosis in the Pediatric Population (STOPP) study was funded by the Canadian Institutes for Health Research (Grant No. CIHR FRN 64285, awarded to LMW, Study Principal Investigator). KP was supported by a Clinical Research Fellowship funded by the Parent Project Muscular Dystrophy and Defeat Duchenne Canada (formerly Jesse’s Journey). LMW was supported by a Tier 1 Clinical Research Chair Award from the University of Ottawa and by the Children’s Hospital of Eastern Ontario Research Institute. MER was supported by a Junior Clinical Research Chair Award from the University of Ottawa and by the Children’s Hospital of Eastern Ontario Research Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have conflicts of interest related to this observational study. Unrelated to the study, some of the authors report the following potential conflicts of interest: NA has participated in clinical trials with Amgen and Novartis. MER has received a study grant from Ascendis Biopharma, and consultancy fees from Ipsen Biopharmaceuticals and Ultragenyx, with funds to her institution. HJM has participated in clinical trials with Roche, PTC Therapeutics, ReveraGen, Catabasis, Novartis and Sarepta, been a consultant for Novartis Gene Therapies and received research support from Roche. UJD has been a consultant to, and participated in clinical trials with, ReveraGen Biopharma. LMW has been a consultant to, and participated in clinical trials with, Amgen, Novartis, PTC, ReveraGen, Catabasis, Ipsen, and Ultragenyx, with funds to her institution.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Phung, K., McAdam, L., Ma, J. et al. Risk factors associated with prevalent vertebral fractures in Duchenne muscular dystrophy. Osteoporos Int 34, 147–160 (2023). https://doi.org/10.1007/s00198-022-06578-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-022-06578-6