Abstract
Summary
TBS algorithm has been updated to account for regional soft tissue noise. In postmenopausal women with osteoporosis, denosumab improved tissue thickness–adjusted TBS vs placebo independently of bone mineral density over 3 years, with the magnitude of changes from baseline or placebo numerically greater than body mass index–adjusted TBS.
Introduction
To evaluate the effect of denosumab on bone microarchitecture assessed by trabecular bone score (TBS) in the FREEDOM study using the updated algorithm that accounts for regional soft tissue thickness (TBSTT) in dual-energy X-ray absorptiometry (DXA) images and to compare percent changes from baseline and placebo with classical body mass index (BMI)–adjusted TBS (TBSBMI).
Methods
Postmenopausal women with lumbar spine or total hip bone mineral density (BMD) T score < − 2.5 and ≥ − 4.0 received placebo or denosumab 60 mg subcutaneously every 6 months. TBSBMI and TBSTT were assessed on lumbar spine DXA scans at baseline and months 1, 12, 24, and 36 in a subset of 279 women (129 placebo, 150 denosumab) who completed the 3-year FREEDOM DXA substudy and rolled over to open-label extension study.
Results
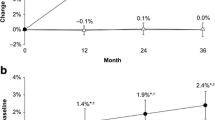
Baseline characteristics were similar between groups. TBSTT in the denosumab group showed numerically greater changes from both baseline and placebo than TBSBMI at months 12, 24, and 36. Denosumab led to progressive increases in BMD (1.2, 5.6, 8.1, and 10.5%) and TBSTT (0.4, 2.3, 2.6, and 3.3%) from baseline to months 1, 12, 24, and 36, respectively. Both TBS changes were significant vs baseline and placebo from months 12 to 36 (p < 0.0001). As expected, BMD and TBSTT were poorly correlated both at baseline and for changes during treatment.
Conclusion
In postmenopausal women with osteoporosis, denosumab significantly improved bone microstructure assessed by TBSTT over 3 years. TBSTT seemed more responsive to denosumab treatment than TBSBMI and was independent of BMD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is characterized by low bone mass and microarchitectural deterioration of the bone tissue, both of which contribute to the risk of fractures [1]. Bone mineral density (BMD) measured by dual-energy X-ray absorptiometry (DXA) explains about 60–80% of bone strength [2] and is used for diagnosis of osteoporosis [3]. Decreased BMD has been well known for many years to be an important predictor of osteoporotic fractures [3]. However, there is considerable overlap in BMD scores between persons with and without osteoporotic fracture, and many fractures occur in persons with non-osteoporotic BMD values (i.e., T score above − 2.5) [1, 4, 5, 6, 7]. These results highlight the importance of other measurements that account for aspects of bone microarchitecture to complement BMD for fracture risk prediction [8, 9].
The trabecular bone score (TBS), which can be extracted from existing DXA or X-ray images, provides an indirect index of trabecular bone microarchitecture [8, 10]. TBS measures the gray-level texture from one pixel to the next across the 2-dimensional (2D) DXA scans using a modified approach of the known experimental variogram. The gray levels measured in the 2D DXA images reflect the variations in X-ray absorption properties in the corresponding 3-dimensional (3D) tissue microarchitecture [8, 10]. A high TBS value is associated with more numerous and connected, and less sparse trabeculae (i.e., better bone structure), whereas a low TBS value indicates a low trabecular number and connectivity, and high trabecular separation (i.e., worse bone structure) [10, 11]. TBS can differentiate 3D microarchitecture properties between 2D DXA scans that present with similar BMD scores [10] and has been shown to predict osteoporotic fractures independently of BMD [8, 12, 13]. TBS has an added predictive value over BMD alone in the estimation of fracture risk [12], and the combination of BMD with TBS can significantly improve the prediction of fractures compared to BMD or TBS alone [8]. In addition, low TBS is strongly associated with many of the clinical risk factors for osteoporotic fractures [14] and predicts fracture risk independently of the Fracture Risk Assessment Tool (FRAX) clinical risk factors. TBS has now been incorporated into FRAX to improve its accuracy to categorize patients at risk for fracture [2, 12, 13, 15, 16, 17].
A technical limitation of both BMD and TBS is the effect of regional soft tissue noise on the DXA images and eventually in the gray-level texture. The latter depends on both the bone and soft tissues, which have different X-ray absorption characteristics. An excess of regional soft tissue thickness/noise tends to attenuate X-ray absorption and decrease gray level variations and subsequently TBS values while increasing BMD values [18]. To account for the regional soft tissue effect on the DXA images, the classical algorithm for computing TBS has been corrected for body mass index (BMI; TBSBMI) [19], a surrogate for soft tissue thickness in the general population with BMI within 15 to 37 kg/m2 [19]. However, BMI is a global measure of excess body fat with limited information on the body morphotype or fat distribution. Because BMI does not provide adiposity information specific for the region of interest, TBSBMI may not accurately correct for the effect of regional soft tissues on TBS, e.g., in individuals with a high versus low lean body mass, or those with abdominal weight accumulation that directly affects TBS derived from lumbar spine DXA versus weight accumulation at other sites [10]. A recent technical update of the TBS algorithm uses a direct built-in correction for soft tissue thickness (TBSTT) measured from the DXA machines. Regional soft tissue thickness provides a direct and specific measurement of the soft tissues that affect the DXA image of the region [20]. TBSTT can better neutralize regional soft tissue noise on the DXA image than TBSBMI [20]. The updated TBSTT algorithm has been shown to be less affected by the artifactual effect of the regional soft tissue presence on the gray-level variogram of the DXA images and is a validated and improved method to evaluate TBS over the previous TBSBMI algorithm [20].
Denosumab is a fully human monoclonal antibody against receptor activator of NF-κB (RANK) ligand that inhibits osteoclast development and function and decreases bone resorption [21, 22, 23, 24]. Denosumab treatment in postmenopausal women with osteoporosis has been shown to increase BMD and decrease the risk of vertebral, non-vertebral, and hip fracture compared with placebo in the phase 3 FREEDOM study and open-label extension (OLE) study [25, 26, 27, 28]. A subset of women in the FREEDOM study participated in a prospective DXA substudy and showed an increase in lumbar spine BMD comparable to that observed in the overall study population [26]. A retrospective analysis of this substudy explored the effect of denosumab on classical TBSBMI and demonstrated that 3 years of denosumab treatment significantly improved TBSBMI versus placebo independently of BMD [29].
This study is a retrospective analysis of lumbar spine DXA scans collected from subjects who enrolled in and completed the FREEDOM DXA substudy and rolled over to the OLE study using the updated TBSTT algorithm with built-in direct correction for regional soft tissue thickness [20]. The objective was to investigate the effect of long-term denosumab on bone microarchitecture as assessed by TBSTT and to compare it with TBSBMI. We hypothesize that the new TBSTT algorithm would improve sensitivity and ability to detect changes resulting from treatment over the classical TBSBMI algorithm. This initial report presents TBS data collected from the lumbar spine DXA scans of subjects during the 3-year DXA substudy. Results from the OLE portion of the study will be reported in a separate publication.
Methods
Study design
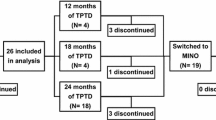
The study design of the FREEDOM (NCT00089791) and OLE (NCT00523341) studies and the DXA substudy have been reported previously [25, 26, 27, 28]. Briefly, the FREEDOM study was an international, multicenter, randomized, double-blind, placebo-controlled study conducted across 213 study centers in 7808 postmenopausal women with osteoporosis. Subjects were randomized to receive either 60 mg of denosumab or placebo subcutaneously every 6 months for 36 months. All subjects received daily supplementation of calcium (≥ 1000 mg) and vitamin D (≥ 400 IU). The prospective DXA substudy included 441 women from 19 centers that performed more frequent BMD assessments and evaluations of additional skeletal sites (Fig. 1). Informed consent was obtained for all subjects who participated in the FREEDOM and OLE studies and the DXA substudy.
A total of 279 subjects completed the 3-year DXA substudy and rolled over to the OLE study. Both TBSBMI and TBSTT data were retrospectively collected from 2409 lumbar spine DXA scans of the 279 subjects during the 3-year DXA substudy (Fig. 1).
Subjects
For the FREEDOM study, eligible subjects were postmenopausal women with a BMD T score between − 4.0 and − 2.5 at the lumbar spine or total hip, naïve to osteoporosis treatment or had received prior bisphosphonate treatment for < 3 years and not within 12 months of study entry, and free of other conditions known to impact bone metabolism. Subjects were required to have a minimum of two evaluable lumbar vertebrae in the L1–L4 region and one evaluable hip (left or right). Subjects with any severe or more than 2 moderate vertebral fractures were excluded [25, 26]. For this study, subjects with BMI > 38 kg/m2 or < 15 kg/m2 were excluded as it was out of the range for a proper assessment of TBSBMI (the same BMI range was applied to TBSTT analysis to allow for an appropriate comparison of the two algorithms). Similarly, subjects were excluded if the DXA scanner or the acquisition mode used for BMD assessment was not compatible with TBS algorithm.
Study assessments
Lumbar spine BMD measurements by GE Healthcare Lunar or Hologic DXA bone densitometers in the DXA substudy were performed at baseline and at months 1, 6, 12, 24, and 36 [26]. The lumbar spine analysis included four vertebrae, L1–L4. Vertebrae were excluded following the International Society of Clinical Densitometry (ISCD) criteria [30]. All DXA scans were centrally read by an imaging vendor (BioClinica [formerly Synarc], Princeton, NJ, USA).
Both TBSTT and TBSBMI were assessed retrospectively on previously obtained lumbar spine DXA scans for each vertebra (L1-L4), in the same region of interest as BMD. The same vertebrae exclusion criteria for BMD analysis [2] were applied for TBS analysis so that an appropriate comparison of the changes in BMD and TBS could be performed. The TBS computation process included two soft tissue compensation techniques: using BMI as a global surrogate of regional soft tissue thickness presence (TBS iNsight software version 3.1 [Medimaps group, Geneva, Switzerland]) and the regional soft tissue thickness itself directly (TBS iNsight software version 4.0 [Medimaps group, Geneva, Switzerland]). Measurement of soft tissue thickness from the DXA scanner is based on physical application of Beer Lambert law regarding attenuation of X-ray, which is the function of density and tissue thickness. Tissue thickness values were extracted directly from the DXA manufacturer software and cross-calibrated to equivalence between Lunar or Hologic scanners. Tissue thickness measurement from the DXA scanner is highly correlated to BMI, with a correlation coefficient of > 0.9. Lumbar spine TBS was calculated as the mean value of the individual TBS of each included vertebra. All the TBS calculations have been performed blinded from clinical outcomes and treatment group allocation. The main analyses of this study were performed using TBSTT, given its advanced accountability of the regional soft tissue effect on the DXA image. TBSBMI was studied and reported here for comparison purposes.
Statistical analyses
The analyses included subjects who completed the DXA substudy and continued to OLE study and had lumbar spine BMD, TBSTT, and TBSBMI measurements at baseline and at least one post-baseline visit during the 3-year DXA substudy. Missing data were not imputed. A repeated measures mixed-effects linear model, adjusting for visit, baseline value, machine type, treatment-by-visit interaction, and baseline value-by-machine type interaction, was used to analyze the percent changes from baseline to follow-up time points in lumbar spine BMD, TBSTT, and TBSBMI. Statistical inferences on differences between the denosumab and placebo groups as well as differences relative to baseline within the denosumab group or the placebo group were assessed at each time point, with no adjustment for multiplicity. The results were reported as least-squares means and associated two-sided 95% confidence intervals (CIs). The percentage of subjects according to TBSTT risk category (TBSTT ≤ 1.027, degraded microarchitecture; TBSTT > 1.027 to ≤ 1.074, partially degraded; TBSTT > 1.074, normal) was summarized at baseline and month 36 in each treatment group. Those thresholds are equivalent to 1.230 and 1.310 usually reported for the classical TBSBMI algorithm (TBSBMI ≤ 1.230, degraded microarchitecture; TBSBMI > 1.230 to ≤ 1.310, partially degraded; TBSBMI > 1.310, normal) because TBSTT thresholds were derived from the same tertile analysis of the same study population from a meta-analysis that generated TBSBMI thresholds [16]. Bhapkar’s test for homogeneity was used to evaluate the significance of the change in the percentage of subjects in each TBSTT risk category from baseline to month 36. The correlation between lumbar spine TBSTT and BMD was evaluated using Pearson correlation coefficients at baseline and for percent change from baseline at each time point within each treatment group. All inferential testing was two-tailed, with p ≤ 0.05 set as the threshold for statistical significance.
Results
A total of 279 (129 placebo, 150 denosumab) postmenopausal women with osteoporosis were included in this analysis (Fig. 1). Baseline characteristics were similar between the placebo and denosumab groups (Table 1). The mean age was ~ 72 years. The mean lumbar spine BMD T score was − 2.8, mean TBSBMI was 1.227, and mean TBSTT was 1.031 in all patients included in the analysis. About 22% of women had a vertebral fracture at baseline. The baseline characteristics of patients in the present analysis were similar to those of the overall population in the FREEDOM study [25] (Supplementary Table 1).
Denosumab was associated with progressive increases from baseline to months 1, 12, 24, and 36 for lumbar spine BMD (LS mean increases of 1.2%, 5.6%, 8.1%, and 10.5%, respectively; all p < 0.0001) and TBSTT (LS mean increases of 0.4%, 2.3%, 2.6%, and 3.3%, respectively; p < 0.0001 from baseline to months 12, 24, and 36) (Fig. 2). The relative increases of the denosumab group vs the placebo group reached 10.4% for lumbar spine BMD and 4.7% for TBSTT at month 36 (p < 0.0001 for both measurements between denosumab vs placebo at months 12, 24, and 36; Fig. 2). The TBSTT scores in the denosumab group showed numerically greater changes from baseline and numerically larger differences from placebo than TBSBMI scores at all post-baseline visits (Table 2).
Denosumab treatment also decreased the number of patients with degraded or partially degraded microarchitecture according to TBSTT (degraded, 48.6% to 35.2%; partially degraded, 25.4% to 17.6%) and increased the number of patients with normal microarchitecture (26.1% to 47.2%) from baseline to month 36 (p < 0.0001; Fig. 3). In contrast, placebo treatment was associated with slightly more patients with degraded or partially degraded microarchitecture and fewer patients with normal microarchitecture from baseline to month 36 (p = 0.0480; Fig. 3).
As expected [29], the lumbar spine BMD and TBSTT were poorly correlated at baseline (Pearson correlation coefficient r2 = 0.34), reinforcing the fact that the two parameters measured different bone properties. Over the course of treatments, change in TBSTT was also poorly correlated with change in BMD. At month 36, correlation coefficient between mean TBSTT and lumbar spine BMD percent change from baseline was 0.08 in the placebo group and 0.06 in the denosumab group (Fig. 4).
Discussion
This retrospective analysis of the DXA substudy in postmenopausal women with osteoporosis showed that denosumab significantly and progressively improved TBSTT vs placebo, independently of BMD over the 3-year treatment period. The magnitude of change in TBSTT from baseline and compared to placebo was numerically greater than that of classical TBSBMI at each post-baseline visit. Bone microstructure, as assessed by TBSTT, improved over 3 years of denosumab treatment vs baseline, with more patients achieving normal microarchitecture and less patients having degraded or partially degraded microarchitecture. These results showed consistent treatment effects of denosumab vs placebo as reported previously [25, 26, 29] and support the improved responsiveness to treatment as measured by the updated TBSTT algorithm.
TBSTT, as expected, was not correlated with lumbar spine BMD at baseline nor over 36 months of treatment with either placebo or denosumab, similar to the lack of correlation between TBSBMI and BMD previously reported in a retrospective analysis of the DXA substudy (r2 ranging from < 0.01 to 0.06) [29]. These results indicate that neutralizing the effect of the soft tissue thickness by the updated TBSTT algorithm did not affect the relationship between TBS and lumbar spine BMD [20]. BMD explains only a small fraction of the variability in TBSTT at baseline (r2 = 0.34) and over the course of 3-year denosumab treatment (r2 = 0.06). In addition, in placebo-treated patients BMD remained constant, whereas TBSTT decreased over 36 months. These results suggest that TBS provides bone information that is not captured by BMD, further confirming TBS and BMD as two independent and complementary assessments of bone status.
TBS is sensitive to the natural progression of osteoporosis and to bone-affecting treatments. The magnitude of change in TBS varies significantly with different therapies [10, 17]. Certain anti-resorptive drugs, such as bisphosphonates (e.g., alendronate, risedronate, ibandronate, zoledronic acid), preserve and increase bone mass, but have not been shown to significantly improve bone structure to date. TBS does demonstrate gains in response to bisphosphonates, but the changes are relatively small, compared to those seen with other anti-resorptive agents, such as denosumab [17, 29, 31]. Moreover, this analysis showed that denosumab significantly improved trabecular bone texture as assessed by TBSTT, decreasing the number of patients with degraded or partially degraded microarchitecture and increasing the number of patients with normal microarchitecture over 3 years of treatment. Denosumab may preserve trabecular microstructure by preventing plate perforation and preserving axially aligned trabeculae [32]. Denosumab may further improve trabecular microstructure by preserving modeling-based bone formation despite its potent inhibition of remodeling [33]. TBS response to the anabolic agent teriparatide was reported at just 6 months after treatment initiation and was of greater amplitude compared to anti-resorptive drugs. TBS increase with teriparatide is currently under investigation for its impact on fracture risk reduction [31].
At present, comparison of treatment effects on TBS between different therapeutic agents should be done with caution. The effect of anti-osteoporotic therapy on TBS has been studied for 36 months or less, and most studies have used the classical TBSBMI algorithm. Not only are studies investigating longer-term treatment effects needed, but also those using TBSTT, instead of TBSBMI, so as to accurately correct for the effect of regional soft tissue presence on the DXA image.
This study is limited by the retrospective nature of the analysis in a small subset (3.6%) of the total FREEDOM study population, which may create imbalances in measured and unmeasured covariates between the two treatment groups. However, the baseline characteristics of patients included in this analysis and mean changes from baseline in lumbar spine BMD over 36 months of treatment in each group were comparable to those reported in the corresponding group of the overall study population [25]. This suggests the observed effect of denosumab on TBSTT likely reflects that of the originally randomized patients in the FREEDOM study. The study results from this analysis may have limited generalizability because mainly women of European ancestry were included. Although the updated TBSTT algorithm is mostly technical (to correct a single noise factor: soft tissue thickness) and unlikely to be affected by gender and/or ethnicity, further studies in more diverse populations will help validate the results reported here.
TBS, derived from existing DXA scans of the lumbar spine, provides clinically relevant information about bone microarchitecture and fracture risk and could assist in monitoring therapeutic responses and guiding management of patients undergoing osteoporosis treatments [8, 10]. This analysis showed that 3-year denosumab treatment in postmenopausal women with osteoporosis was associated with a significant and progressive increase in regional tissue thickness–adjusted TBS, reflecting improved bone microstructure, as compared with placebo and baseline, with numerically greater responsiveness to treatment than BMI-adjusted TBS. Both TBS measurements were poorly correlated with BMD at baseline. Changes in both TBS measurements and BMD from baseline were also poorly correlated at each time point over 3 years of treatment, as shown in this and previous analyses [29]. These results, together with previous observations that TBS predicts fracture risk independently of BMD [8, 12, 13, 34], support the usefulness of the updated TBSTT algorithm as a practical tool, in addition to BMD, to monitor osteoporotic patients under denosumab treatment in routine clinical practice. The effect of long-term denosumab treatment for up to 10 years on bone microarchitecture will be reported later, based on TBSTT data collected from the OLE portion of the FREEDOM TBS post hoc analysis.
Data availability
Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: https://wwwext.amgen.com/science/clinical-trials/clinical-data-transparency-practices/clinical-trial-data-sharing-request/
References
Schuit SC, van der Klift M, Weel AE, de Laet CE, Burger H, Seeman E et al (2004) Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone 34(1):195–202. https://doi.org/10.1016/j.bone.2003.10.001
Silva BC, Broy SB, Boutroy S, Schousboe JT, Shepherd JA, Leslie WD (2015) Fracture risk prediction by non-BMD DXA measures: the 2015 ISCD Official Positions Part 2: trabecular bone score. J Clin Densitom 18(3):309–330. https://doi.org/10.1016/j.jocd.2015.06.008
Nuti R, Brandi ML, Checchia G, Di Munno O, Dominguez L, Falaschi P et al (2019) Guidelines for the management of osteoporosis and fragility fractures. Intern Emerg Med 14(1):85–102. https://doi.org/10.1007/s11739-018-1874-2
Hordon LD, Raisi M, Aaron JE, Paxton SK, Beneton M, Kanis JA (2000) Trabecular architecture in women and men of similar bone mass with and without vertebral fracture: II. Three-dimensional histology. Bone 27(2):277–282. https://doi.org/10.1016/s8756-3282(00)00328-8
Stone KL, Seeley DG, Lui LY, Cauley JA, Ensrud K, Browner WS, Nevitt MC, Cummings SR (2003) BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res 18(11):1947–1954. https://doi.org/10.1359/jbmr.2003.18.11.1947
Siris ES, Chen YT, Abbott TA, Barrett-Connor E, Miller PD, Wehren LE, Berger ML (2004) Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med 164(10):1108–1112. https://doi.org/10.1001/archinte.164.10.1108
Cranney A, Jamal SA, Tsang JF, Josse RG, Leslie WD (2007) Low bone mineral density and fracture burden in postmenopausal women. CMAJ 177(6):575–580. https://doi.org/10.1503/cmaj.070234
Hans D, Goertzen AL, Krieg MA, Leslie WD (2011) Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. J Bone Miner Res 26(11):2762–2769. https://doi.org/10.1002/jbmr.499
Topoliński T, Mazurkiewicz A, Jung S, Cichański A, Nowicki K (2012) Microarchitecture parameters describe bone structure and its strength better than BMD. Sci World J 2012:502781. https://doi.org/10.1100/2012/502781
Silva BC, Leslie WD, Resch H, Lamy O, Lesnyak O, Binkley N et al (2014) Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res 29(3):518–530. https://doi.org/10.1002/jbmr.2176
Muschitz C, Kocijan R, Haschka J, Pahr D, Kaider A, Pietschmann P et al (2015) TBS reflects trabecular microarchitecture in premenopausal women and men with idiopathic osteoporosis and low-traumatic fractures. Bone 79:259–266. https://doi.org/10.1016/j.bone.2015.06.007
Winzenrieth R, Dufour R, Pothuaud L, Hans D (2010) A retrospective case-control study assessing the role of trabecular bone score in postmenopausal Caucasian women with osteopenia: analyzing the odds of vertebral fracture. Calcif Tissue Int 86(2):104–109. https://doi.org/10.1007/s00223-009-9322-y
McCloskey EV, Odén A, Harvey NC, Leslie WD, Hans D, Johansson H et al (2015) Adjusting fracture probability by trabecular bone score. Calcif Tissue Int 96(6):500–509. https://doi.org/10.1007/s00223-015-9980-x
Leslie WD, Krieg MA, Hans D, Manitoba Bone Density Program (2013) Clinical factors associated with trabecular bone score. J Clin Densitom 16(3):374–379. https://doi.org/10.1016/j.jocd.2013.01.006
Leslie WD, Johansson H, Kanis JA, Lamy O, Oden A, McCloskey EV, Hans D (2014) Lumbar spine texture enhances 10-year fracture probability assessment. Osteoporos Int 25(9):2271–2277. https://doi.org/10.1007/s00198-014-2761-y
McCloskey EV, Odén A, Harvey NC, Leslie WD, Hans D, Johansson H et al (2016) A meta-analysis of trabecular bone score in fracture risk prediction and its relationship to FRAX. J Bone Miner Res 31(5):940–948. https://doi.org/10.1002/jbmr.2734
Hans D, Šteňová E, Lamy O (2017) The trabecular bone score (TBS) complements DXA and the FRAX as a fracture risk assessment tool in routine clinical practice. Curr Osteoporos Rep 15(6):521–531. https://doi.org/10.1007/s11914-017-0410-z
Amnuaywattakorn S, Sritara C, Utamakul C, Chamroonrat W, Kositwattanarerk A, Thamnirat K et al (2016) Simulated increased soft tissue thickness artefactually decreases trabecular bone score: a phantom study. BMC Musculoskelet Disord 17:17. https://doi.org/10.1186/s12891-016-0886-1
Schacter GI, Leslie WD, Majumdar SR, Morin SN, Lix LM, Hans D (2017) Clinical performance of an updated trabecular bone score (TBS) algorithm in men and women: the Manitoba BMD cohort. Osteoporos Int 28(11):3199–3203. https://doi.org/10.1007/s00198-017-4166-1
Shevroja E, Aubry-Rozier B, Hans G, Gonzalez-Rodriguez E, Stoll D, Lamy O, Hans D (2019) Clinical performance of the updated trabecular bone score (TBS) algorithm, which accounts for the soft tissue thickness: The Osteolaus study. J Bone Miner Res 34(12):2229–2237. https://doi.org/10.1002/jbmr.3851
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T et al (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93(2):165–176. https://doi.org/10.1016/s0092-8674(00)81569-x
Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S et al (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci 95(7):3597–3602. https://doi.org/10.1073/pnas.95.7.3597
Lacey DL, Tan HL, Lu J, Kaufman S, Van G, Qiu W et al (2000) Osteoprotegerin ligand modulates murine osteoclast survival in vitro and in vivo. Am J Pathol 157(2):435–448. https://doi.org/10.1016/S0002-9440(10)64556-7
Udagawa N, Takahashi N, Yasuda H, Mizuno A, Itoh K, Ueno Y et al (2000) Osteoprotegerin produced by osteoblasts is an important regulator in osteoclast development and function. Endocrinology 141(9):3478–3484. https://doi.org/10.1210/endo.141.9.7634
Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR et al (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361(8):756–765. https://doi.org/10.1056/NEJMoa0809493
Bolognese MA, Teglbjærg CS, Zanchetta JR, Lippuner K, McClung MR, Brandi ML et al (2013) Denosumab significantly increases DXA BMD at both trabecular and cortical sites: results from the FREEDOM study. J Clin Densitom 16(2):147–153. https://doi.org/10.1016/j.jocd.2012.02.006
Bone HG, Wagman RB, Brandi ML, Brown JP, Chapurlat R, Cummings SR et al (2017) 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 5(7):513–523. https://doi.org/10.1016/S2213-8587(17)30138-9
Ferrari S, Butler PW, Kendler DL, Miller PD, Roux C, Wang AT, Huang S, Wagman RB, Lewiecki EM (2019) Further nonvertebral fracture reduction beyond 3 years for up to 10 years of denosumab treatment. J Clin Endocrinol Metab 104(8):3450–3461. https://doi.org/10.1210/jc.2019-00271
McClung MR, Lippuner K, Brandi ML, Zanchetta JR, Bone HG, Chapurlat R, Hans D, Wang A, Zapalowski C, Libanati C (2017) Effect of denosumab on trabecular bone score in postmenopausal women with osteoporosis. Osteoporos Int 28(10):2967–2973. https://doi.org/10.1007/s00198-017-4140-y
Shuhart CR, Yeap SS, Anderson PA, Jankowski LG, Lewiecki EM, Morse LR et al (2019) Executive summary of the 2019 ISCD position development conference on monitoring treatment, DXA cross-calibration and least significant change, spinal cord injury, peri-prosthetic and orthopedic bone health, transgender medicine, and pediatrics. J Clin Densitom 22(4):453–471. https://doi.org/10.1016/j.jocd.2019.07.001
Di Gregorio S, Del Rio L, Rodriguez-Tolra J, Bonel E, García M, Winzenrieth R (2015) Comparison between different bone treatments on areal bone mineral density (aBMD) and bone microarchitectural texture as assessed by the trabecular bone score (TBS). Bone 75:138–143. https://doi.org/10.1016/j.bone.2014.12.062
Hu YJ, Chines A, Shi Y, Seeman E, Guo XE (2022) The effect of denosumab and alendronate on trabecular plate and rod microstructure at the distal tibia and radius: a post hoc HR-pQCT study. Bone 154:116187. https://doi.org/10.1016/j.bone.2021.116187
Dempster DW, Chines A, Bostrom MP, Nieves JW, Zhou H, Chen L et al (2020) Modeling-based bone formation in the human femoral neck in subjects treated with denosumab. J Bone Miner Res 35(7):1282–1288. https://doi.org/10.1002/jbmr.4006
Shevroja E, Lamy O, Kohlmeier L, Koromani F, Rivadeneira F, Hans D (2017) Use of trabecular bone score (TBS) as a complementary approach to dual-energy X-ray absorptiometry (DXA) for fracture risk assessment in clinical practice. J Clin Densitom 20(3):334–345. https://doi.org/10.1016/j.jocd.2017.06.019
Acknowledgements
This study was sponsored by Amgen Inc., Thousand Oaks, CA, USA. Jinling Wu (BioScience Communications, New York, NY) provided writing and editorial support, which was funded by Amgen Inc.
Funding
Open access funding provided by University of Lausanne This study was funded by Amgen Inc., Thousand Oaks, CA, USA.
Author information
Authors and Affiliations
Contributions
Study concept and design: Didier Hans, Michael McClung, Michele McDermott, Shuang Huang, Min Kim.
Data collection: Didier Hans (blind from clinical outcomes and treatment groups), Shuang Huang, and Enisa Shevroja (blind from clinical outcomes and treatment groups).
Analysis and interpretation of the data: All authors.
Statistical expertise: Shuang Huang.
Drafting and critical review/revision of the article: All authors.
The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study complied with the principles of the Declaration of Helsinki. Institutional review boards and ethics committees approved the protocol and consent process. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Code availability
Not applicable.
Conflict of interest
Didier Hans is the co-owner of the TBS patent and has corresponding ownership shares and position at Medimaps group.
Enisa Shevroja has no conflict of interest.
Michael McClung received honorarium and consulting fees from Amgen.
Michele McDermott is an employee and stockholder of Amgen.
Shuang Huang is an employee and stockholder of Amgen.
Min Kim is an employee and stockholder of Amgen.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hans, D., Shevroja, E., McDermott, M. et al. Updated trabecular bone score accounting for the soft tissue thickness (TBSTT) demonstrated significantly improved bone microstructure with denosumab in the FREEDOM TBS post hoc analysis. Osteoporos Int 33, 2517–2525 (2022). https://doi.org/10.1007/s00198-022-06549-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-022-06549-x