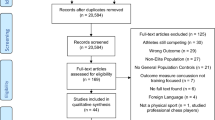
Abstract
Summary
Twenty men with spinal cord injury (SCI) were randomized into two 16-week intervention groups receiving testosterone treatment (TT) or TT combined with resistance training (TT + RT). TT + RT appears to hold the potential to reverse or slow down bone loss following SCI if provided over a longer period.
Introduction
Persons with SCI experience bone loss below the level of injury. The combined effects of resistance training and TT on bone quality following SCI remain unknown.
Methods
Men with SCI were randomized into 16-week treatments receiving TT or TT + RT. Magnetic resonance imaging (MRI) of the right lower extremity before participation and post-intervention was used to visualize the proximal, middle, and distal femoral shaft, the quadriceps tendon, and the intermuscular fascia of the quadriceps. For the TT + RT group, MRI microarchitecture techniques were utilized to elucidate trabecular changes around the knee. Individual mixed models were used to estimate effect sizes.
Results
Twenty participants completed the pilot trial. A small effect for yellow marrow in the distal femur was indicated as increases following TT and decreases following TT + RT were observed. Another small effect was observed as the TT + RT group displayed greater increases in intermuscular fascia length than the TT arm. Distal femur trabecular changes for the TT + RT group were generally small in effect (decreased trabecular thickness variability, spacing, and spacing variability; increased network area). Medium effects were generally observed in the proximal tibia (increased plate width, trabecular thickness, and network area; decreased trabecular spacing and spacing variability).
Conclusions
This pilot suggests longer TT + RT interventions may be a viable rehabilitation technique to combat bone loss following SCI.
Clinical trial registration
Registered with clinicaltrials.gov: NCT01652040 (07/27/2012).



Similar content being viewed by others
Data availability
The corresponding author will make the data available upon request.
References
Modlesky CM, Slade JM, Bickel CS, Meyer RA, Dudley GA (2005) Deteriorated geometric structure and strength of the midfemur in men with complete spinal cord injury. Bone 36:331–339. https://doi.org/10.1016/j.bone.2004.10.012
Eser P, Frotzler A, Zehnder Y, Wick L, Knecht H, Denoth J, Schiessl H (2004) Relationship between the duration of paralysis and bone structure: a pQCT study of spinal cord injured individuals. Bone 34:869–880. https://doi.org/10.1016/j.bone.2004.01.001
Cirnigliaro CM, Myslinski MJ, La Fountaine MF et al (2017) Bone loss at the distal femur and proximal tibia in persons with spinal cord injury: imaging approaches, risk of fracture, and potential treatment options. Osteoporos Int 28:747–765. https://doi.org/10.1007/s00198-016-3798-x
Bauman WA, Cardozo CP (2015) Osteoporosis in individuals with spinal cord injury. PM R 7:188–201. https://doi.org/10.1016/j.pmrj.2014.08.948
Garland DE, Adkins RH, Kushwaha V, Stewart C (2004) Risk factors for osteoporosis at the knee in the spinal cord injury population. J Spinal Cord Med 27:202–206. https://doi.org/10.1080/10790268.2004.11753748
Tan CO, Battaglino RA, Morse LR (2013) Spinal cord injury and osteoporosis: causes, mechanisms, and rehabilitation strategies. Int J Phys Med Rehabil 1:1–10. https://doi.org/10.4172/2329-9096.1000127
Gorgey AS, Poarch HJ, Adler RA, Khalil RE, Gater DR (2013) Femoral bone marrow adiposity and cortical bone cross-sectional areas in men with motor complete spinal cord injury. PM&R 5:939–948. https://doi.org/10.1016/j.pmrj.2013.05.006
Zügel M, Maganaris CN, Wilke J, Jurkat-Rott K, Klingler W, Wearing SC, Findley T, Barbe MF, Steinacker JM, Vleeming A, Bloch W, Schleip R, Hodges PW (2018) Fascial tissue research in sports medicine: from molecules to tissue adaptation, injury and diagnostics: consensus statement. Br J Sports Med 52:1497. https://doi.org/10.1136/bjsports-2018-099308
Maganaris CN, Reeves ND, Rittweger J, Sargeant AJ, Jones DA, Gerrits K, de Haan A (2006) Adaptive response of human tendon to paralysis. Muscle Nerve 33:85–92. https://doi.org/10.1002/mus.20441
Gorgey A, Khalil R (2015) Neuromuscular electrical stimulation training increases intermuscular fascial length but not tendon cross-sectional area after spinal cord injury. Top Spinal Cord Inj Rehabil 21:87–92. https://doi.org/10.1310/sci2101-87
Bauman WA, Emmons RR, Cirnigliaro CM, Kirshblum SC, Spungen AM (2011) An effective oral vitamin D replacement therapy in persons with spinal cord injury. J Spinal Cord Med 34:455–460. https://doi.org/10.1179/2045772311Y.0000000032
Yarrow JF, Phillips EG, Conover CF, Bassett TE, Chen C, Teurlings T, Vasconez A, Alerte J, Prock H, Jiron JM, Flores M, Aguirre JI, Borst SE, Ye F (2017) Testosterone plus finasteride prevents bone loss without prostate growth in a rodent spinal cord injury model. J Neurotrauma 34:2972–2981. https://doi.org/10.1089/neu.2016.4814
Nightingale TE, Moore P, Harman J, Khalil R, Gill RS, Castillo T, Adler RA, Gorgey AS (2017) Body composition changes with testosterone replacement therapy following spinal cord injury and aging. A Mini Review. J Spinal Cord Med 41:1–21. https://doi.org/10.1080/10790268.2017.1357917
Abilmona SM, Sumrell RM, Gill RS, Adler RA, Gorgey AS (2019) Serum testosterone levels may influence body composition and cardiometabolic health in men with spinal cord injury. Spinal Cord 57:229–239. https://doi.org/10.1038/s41393-018-0207-7
Gordon KE, Wald MJ, Schnitzer TJ (2013) Effect of parathyroid hormone combined with gait training on bone density and bone architecture in people with chronic spinal cord injury. PM R 5:663–671. https://doi.org/10.1016/j.pmrj.2013.03.032
Varghese SM, Chandy BR, Thomas R, Tharion G (2016) Effect of zoledronic acid on osteoporosis after chronic spinal cord injury: a randomized controlled trial. Crit Rev Phys Rehabil Med 28:85–93. https://doi.org/10.1615/CritRevPhysRehabilMed.2016018768
Gifre L, Vidal J, Carrasco JL, Muxi A, Portell E, Monegal A, Guañabens N, Peris P (2016) Denosumab increases sublesional bone mass in osteoporotic individuals with recent spinal cord injury. Osteoporos Int 27:405–410. https://doi.org/10.1007/s00198-015-3333-5
Gilchrist NL, Frampton CM, Acland RH, Nicholls MG, March RL, Maguire P, Heard A, Reilly P, Marshall K (2007) Alendronate prevents bone loss in patients with acute spinal cord injury: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab 92:1385–1390. https://doi.org/10.1210/jc.2006-2013
Cirnigliaro CM, La Fountaine MF, Parrott JS et al (2020) Administration of denosumab preserves bone mineral density at the knee in persons with subacute spinal cord injury: findings from a randomized clinical trial. JBMR Plus 4:1–11. https://doi.org/10.1002/jbm4.10375
Chen SC, Lai CH, Chan WP, Huang MH, Tsai HW, Chen JJJ (2005) Increases in bone mineral density after functional electrical stimulation cycling exercises in spinal cord injured patients. Disabil Rehabil 27:1337–1341. https://doi.org/10.1080/09638280500164032
Frotzler A, Coupaud S, Perret C, Kakebeeke TH, Hunt KJ, Donaldson NN, Eser P (2008) High-volume FES-cycling partially reverses bone loss in people with chronic spinal cord injury. Bone 43:169–176. https://doi.org/10.1016/j.bone.2008.03.004
Dudley-Javoroski S, Saha PK, Liang G, Li C, Gao Z, Shields RK (2012) High dose compressive loads attenuate bone mineral loss in humans with spinal cord injury. Osteoporos Int 23:2335–2346. https://doi.org/10.1007/s00198-011-1879-4
Dudley-Javoroski S, Shields RK (2008) Asymmetric bone adaptations to soleus mechanical loading after spinal cord injury. J Musculoskelet Neuronal Interact 8:227–238
Gorgey AS, Mather KJ, Cupp HR, Gater DR (2012) Effects of resistance training on adiposity and metabolism after spinal cord injury. Med Sci Sports Exerc 44:165–174. https://doi.org/10.1249/MSS.0b013e31822672aa
Groah SL, Lichy AM, Libin AV, Ljungberg I (2010) Intensive electrical stimulation attenuates femoral bone loss in acute spinal cord injury. PM R 2:1080–1087. https://doi.org/10.1016/j.pmrj.2010.08.003
Bélanger M, Stein RB, Wheeler GD, Gordon T, Leduc B (2000) Electrical stimulation: can it increase muscle strength and reverse osteopenia in spinal cord injured individuals? Arch Phys Med Rehabil 81:1090–1098. https://doi.org/10.1053/apmr.2000.7170
Mahoney ET, Bickel CS, Elder C, Black C, Slade JM, Apple D Jr, Dudley GA (2005) Changes in skeletal muscle size and glucose tolerance with electrically stimulated resistance training in subjects with chronic spinal cord injury. Arch Phys Med Rehabil 86:1502–1504. https://doi.org/10.1016/j.apmr.2004.12.021
Otzel DM, Lee J, Ye F, Borst S, Yarrow J (2018) Activity-based physical rehabilitation with adjuvant testosterone to promote neuromuscular recovery after spinal cord injury. Int J Mol Sci 19:1–36. https://doi.org/10.3390/ijms19061701
Yarrow JF, Kok HJ, Phillips EG, Conover CF, Lee J, Bassett TE, Buckley KH, Reynolds MC, Wnek RD, Otzel DM, Chen C, Jiron JM, Graham ZA, Cardozo C, Vandenborne K, Bose PK, Aguirre JI, Borst SE, Ye F (2019) Locomotor training with adjuvant testosterone preserves cancellous bone and promotes muscle plasticity in male rats after severe spinal cord injury. J Neurosci Res 98:1–26. https://doi.org/10.1002/jnr.24564
Biering-Sørensen F, Hansen B, Lee BSB (2009) Non-pharmacological treatment and prevention of bone loss after spinal cord injury: a systematic review. Spinal Cord 47:508–518. https://doi.org/10.1038/sc.2008.177
Morse LR, Biering-Soerensen F, Carbone LD, Cervinka T, Cirnigliaro CM, Johnston TE, Liu N, Troy KL, Weaver FM, Shuhart C, Craven BC (2019) Bone mineral density testing in spinal cord injury: 2019 ISCD official position. J Clin Densitom 22:554–566. https://doi.org/10.1016/j.jocd.2019.07.012
Gorgey AS, Dudley GA (2007) Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord 45:304–309. https://doi.org/10.1038/sj.sc.3101968
Saha PK, Chaudhuri BB (1996) 3D digital topology under binary transformation with applications. Comput Vis Image Underst 63:418–429. https://doi.org/10.1006/cviu.1996.0032
Chen C, Jin D, Liu Y, Wehrli FW, Chang G, Snyder PJ, Regatte RR, Saha PK (2016) Trabecular bone characterization on the continuum of plates and rods using in vivo MR imaging and volumetric topological analysis. Phys Med Biol 61:N478–N496. https://doi.org/10.1088/0031-9155/61/18/N478
Chang G, Rajapakse CS, Chen C, Welbeck A, Egol K, Regatte RR, Saha PK, Honig S (2018) 3-T MR imaging of proximal femur microarchitecture in subjects with and without fragility fracture and nonosteoporotic proximal femur bone mineral density. Radiology 287:608–619. https://doi.org/10.1148/radiol.2017170138
Gomberg BR, Wehrli FW, Vasilić B, Weening RH, Saha PK, Song HK, Wright AC (2004) Reproducibility and error sources of μ-MRI-based trabecular bone structural parameters of the distal radius and tibia. Bone 35:266–276. https://doi.org/10.1016/j.bone.2004.02.017
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd. Lawrence Erlbaum Associates, Hillsdale
Bender R, Lange S (2001) Adjusting for multiple testing - when and how? J Clin Epidemiol 54:343–349. https://doi.org/10.1016/S0895-4356(00)00314-0
Szucs D, Ioannidis JPA (2017) When null hypothesis significance testing is unsuitable for research: a reassessment. Front Hum Neurosci 11. https://doi.org/10.3389/fnhum.2017.00390
Johnston TE, Marino RJ, Oleson CV, Schmidt-Read M, Leiby BE, Sendecki J, Singh H, Modlesky CM (2016) Musculoskeletal effects of 2 functional electrical stimulation cycling paradigms conducted at different cadences for people with spinal cord injury: a pilot study. Arch Phys Med Rehabil 97:1413–1422. https://doi.org/10.1016/j.apmr.2015.11.014
Gibbons RS, Beaupre GS, Kazakia GJ (2016) FES-rowing attenuates bone loss following spinal cord injury as assessed by HR-pQCT. Spinal Cord Ser Cases 2:1–4. https://doi.org/10.1038/scsandc.2015.41
Johnston BD, Johnston JD, Cooper DML et al (2016) Cortical bone porosity: what is it, why is it important, and how can we detect it? Curr Osteoporos Rep 14:187–198. https://doi.org/10.1007/s11914-016-0319-y
Dudley-Javoroski S, Petrie MA, McHenry CL et al (2016) Bone architecture adaptations after spinal cord injury: impact of long-term vibration of a constrained lower limb. Osteoporos Int 27:1149–1160. https://doi.org/10.1007/s00198-015-3326-4
Yarrow JF, Conover CF, Beggs LA, Beck DT, Otzel DM, Balaez A, Combs SM, Miller JR, Ye F, Aguirre JI, Neuville KG, Williams AA, Conrad BP, Gregory CM, Wronski TJ, Bose PK, Borst SE (2014) Testosterone dose dependently prevents bone and muscle loss in rodents after spinal cord injury. J Neurotrauma 31:834–845. https://doi.org/10.1089/neu.2013.3155
Guzzoni V, Selistre-de-Araújo H, de Cássia Marqueti R (2018) Tendon remodeling in response to resistance training, anabolic androgenic steroids and aging. Cells 7:251. https://doi.org/10.3390/cells7120251
Beggs LA, Ye F, Ghosh P, Beck DT, Conover CF, Balaez A, Miller JR, Phillips EG, Zheng N, Williams AA, Aguirre JI, Wronski TJ, Bose PK, Borst SE, Yarrow JF (2015) Sclerostin inhibition prevents spinal cord injury-induced cancellous bone loss. J Bone Miner Res 30:681–689. https://doi.org/10.1002/jbmr.2396
Holman ME, Gorgey AS (2019) Testosterone and resistance training improve muscle quality in spinal cord injury. Med Sci Sports Exerc 51:1591–1598. https://doi.org/10.1249/MSS.0000000000001975
Wilk KE, Escamilla RF, Fleisig GS, Barrentine SW, Andrews JR, Boyd ML (1996) A comparison of tibiofemoral joint forces and electromyographic activity during open and closed kinetic chain exercises. Am J Sports Med 24:518–527. https://doi.org/10.1177/036354659602400418
Mesfar W, Shirazi-Adl A (2008) Computational biomechanics of knee joint in open kinetic chain extension exercises. Comput Methods Biomech Biomed Engin 11:55–61. https://doi.org/10.1080/10255840701552028
McHenry CL, Shields RK (2012) A biomechanical analysis of exercise in standing, supine, and seated positions: implications for individuals with spinal cord injury. J Spinal Cord Med 35:140–147. https://doi.org/10.1179/2045772312Y.0000000011
Gorgey AS, Khalil RE, Gill R, Gater DR, Lavis TD, Cardozo CP, Adler RA (2019) Low-dose testosterone and evoked resistance exercise after spinal cord injury on cardio-metabolic risk factors: an open-label randomized clinical trial. J Neurotrauma 36:2631–2645. https://doi.org/10.1089/neu.2018.6136
Dolbow DR, Gorgey AS, Daniels JA, Adler RA, Moore JR, Gater DR (2011) The effects of spinal cord injury and exercise on bone mass: a literature review. NeuroRehabilitation 29:261–269. https://doi.org/10.3233/NRE-2011-0702
Acknowledgments
This work was supported by the United States Department of Veterans Affairs (VA-RRD CDA2). We thank the participants and the research staff who made the current study possible. We also thank the Hunter Holmes McGuire Research Institute and the Spinal Cord Injury and Disorders Services for providing the environment to conduct the clinical research.
Funding
The study was funded by the United States Department of Veterans Affairs: VA-RRD CDA2 (B7867-W).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Code availability
Custom software was used for computing trabecular bone measures from magnetic resonance images. Other software utilized is commercially available.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Holman, M., Chang, G., Ghatas, M. et al. Bone and non-contractile soft tissue changes following open kinetic chain resistance training and testosterone treatment in spinal cord injury: an exploratory study. Osteoporos Int 32, 1321–1332 (2021). https://doi.org/10.1007/s00198-020-05778-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-020-05778-2